CD8α Structural Domains Enhance GUCY2C CAR-T Cell Efficacy
- PMID: 39315411
- PMCID: PMC11423665
- DOI: 10.1080/15384047.2024.2398801
CD8α Structural Domains Enhance GUCY2C CAR-T Cell Efficacy
Abstract
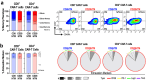
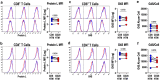
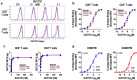
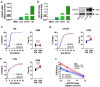
Despite success in treating some hematological malignancies, CAR-T cells have not yet produced similar outcomes in solid tumors due, in part, to the tumor microenvironment, poor persistence, and a paucity of suitable target antigens. Importantly, the impact of the CAR components on these challenges remains focused on the intracellular signaling and antigen-binding domains. In contrast, the flexible hinge and transmembrane domains have been commoditized and are the least studied components of the CAR. Here, we compared the hinge and transmembrane domains derived from either the CD8ɑ or CD28 molecule in identical GUCY2C-targeted third-generation designs for colorectal cancer. While these structural domains do not contribute to differences in antigen-independent contexts, such as CAR expression and differentiation and exhaustion phenotypes, the CD8ɑ structural domain CAR has a greater affinity for GUCY2C. This results in increased production of inflammatory cytokines and granzyme B, improved cytolytic effector function with low antigen-expressing tumor cells, and robust anti-tumor efficacy in vivo compared with the CD28 structural domain CAR. This suggests that CD8α structural domains should be considered in the design of all CARs for the generation of high-affinity CARs and optimally effective CAR-T cells in solid tumor immunotherapy.
Keywords: CAR-T cell therapy; Chimeric antigen receptor; GUCY2C; colorectal cancer.
Conflict of interest statement
T.R. Baybutt reports pending patents related to the submitted work; and CAR-T-related patents licensed to TDT. S.A.W. is a member of the Board of Directors for Targeted Diagnostics & Therapeutics, Inc. which provided research funding that, in part, supported this work and has a license to commercialize inventions related to this work. A.E. Snook reports personal fees from Targeted Diagnostics and Therapeutics (TDT), Inc. and Vittoria Biotherapeutics Inc outside the submitted work; pending patents related to the submitted work; and CAR-T-related patents licensed to TDT.
Figures



References
-
- Quintanilha JCF, Graf RP, Fisher VA, Oxnard GR, Ellis H, Panarelli N, Lin DI, Li G, Huang RSP, Ross JS, et al. Comparative effectiveness of immune checkpoint inhibitors vs chemotherapy in patients with metastatic colorectal cancer with measures of microsatellite instability, mismatch repair, or tumor mutational burden. JAMA Netw Open. 2023. Jan 3;6(1):e2252244. doi:10.1001/jamanetworkopen.2022.52244. - DOI - PMC - PubMed
-
- Parkhurst M, Goff SL, Lowery FJ, Beyer RK, Halas H, Robbins PF, Prickett TD, Gartner JJ, Sindiri S, Krishna S, et al. Adoptive transfer of personalized neoantigen-reactive tcr-transduced T cells in metastatic colorectal cancer: phase 2 trial interim results. Nat Med. 2024. Jul 11. doi:10.1038/s41591-024-03109-0. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
