Human Alzheimer's Disease ATN/ABC Staging Applied to Aging Rhesus Macaque Brains: Association With Cognition and MRI-Based Regional Gray Matter Volume
- PMID: 39315417
- PMCID: PMC11451939
- DOI: 10.1002/cne.25670
Human Alzheimer's Disease ATN/ABC Staging Applied to Aging Rhesus Macaque Brains: Association With Cognition and MRI-Based Regional Gray Matter Volume
Abstract
The brain changes of Alzheimer's disease (AD) include Abeta (Aβ) amyloid plaques ("A"), abnormally phosphorylated tau tangles ("T"), and neurodegeneration ("N"). These have been used to construct in vivo and postmortem diagnostic and staging classifications for evaluating the spectrum of AD in the "ATN" and "ABC" ("B" for Braak tau stage, "C" for Consortium to Establish a Registry for Alzheimer's Disease [CERAD] neuritic plaque density) systems. Another common AD feature involves cerebral amyloid angiopathy (CAA). We report the first experiment to examine relationships among cognition, brain distribution of amyloid plaques, CAA, tau/tangles, and magnetic resonance imaging (MRI)-determined volume changes (as a measure of "N") in the same group of behaviorally characterized nonhuman primates. Both ATN and ABC systems were applied to a group of 32 rhesus macaques aged between 7 and 33 years. When an immunohistochemical method for "T" and "B" was used, some monkeys were "triple positive" on ATN, with a maximum ABC status of A1B2C3. With silver or thioflavin S methods, however, all monkeys were classified as T-negative and B0, indicating the absence of mature neurofibrillary tangles (NFTs) and hence neuropathologically defined AD. Although monkeys at extremes of the ATN and ABC classifications, or with frequent CAA, had significantly lower scores on some cognitive tests, the lack of fully mature NFTs or dementia-consistent cognitive impairment indicates that fully developed AD may not occur in rhesus macaques. There were sex differences noted in the types of histopathology present, and only CAA was significantly related to gray matter volume.
Keywords: RRID:AB_223647; RRID:AB_2564652; aging; amyloid plaques; phosphorylated tau (ptau).
© 2024 Wiley Periodicals LLC.
Figures
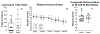
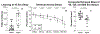
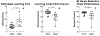
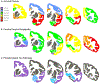




Similar articles
-
Cerebral Amyloid Angiopathy, Dementia, and Alzheimer Neuropathologic Changes: Findings From the ACT Autopsy Cohort.Neurology. 2024 Nov 26;103(10):e210009. doi: 10.1212/WNL.0000000000210009. Epub 2024 Oct 31. Neurology. 2024. PMID: 39481068
-
Ante-mortem cognitive trajectories associated with Aβ and tau biomarker profiles in older adults with cerebrovascular disease: a longitudinal cohort study.Alzheimers Res Ther. 2025 Jul 18;17(1):165. doi: 10.1186/s13195-025-01776-w. Alzheimers Res Ther. 2025. PMID: 40682084 Free PMC article.
-
CSF tau and the CSF tau/ABeta ratio for the diagnosis of Alzheimer's disease dementia and other dementias in people with mild cognitive impairment (MCI).Cochrane Database Syst Rev. 2017 Mar 22;3(3):CD010803. doi: 10.1002/14651858.CD010803.pub2. Cochrane Database Syst Rev. 2017. PMID: 28328043 Free PMC article.
-
Amyloid-β peptide signature associated with cerebral amyloid angiopathy in familial Alzheimer's disease with APPdup and Down syndrome.Acta Neuropathol. 2024 Jul 18;148(1):8. doi: 10.1007/s00401-024-02756-4. Acta Neuropathol. 2024. PMID: 39026031 Free PMC article.
-
Plasma and cerebrospinal fluid amyloid beta for the diagnosis of Alzheimer's disease dementia and other dementias in people with mild cognitive impairment (MCI).Cochrane Database Syst Rev. 2014 Jun 10;2014(6):CD008782. doi: 10.1002/14651858.CD008782.pub4. Cochrane Database Syst Rev. 2014. PMID: 24913723 Free PMC article.
Cited by
-
Tau and tauopathies across primate species: implications for modeling neurodegenerative disorders.Front Aging Neurosci. 2025 Jul 23;17:1598245. doi: 10.3389/fnagi.2025.1598245. eCollection 2025. Front Aging Neurosci. 2025. PMID: 40771194 Free PMC article. Review.
-
Expression patterns of blood-based biomarkers of neurodegeneration and inflammation across adulthood in rhesus macaques.Exp Gerontol. 2025 May;203:112736. doi: 10.1016/j.exger.2025.112736. Epub 2025 Mar 21. Exp Gerontol. 2025. PMID: 40122475 Free PMC article.
References
-
- Alexander GE, Chen K, Aschenbrenner M, Merkley TL, Santerre-Lemmon LE, Shamy JL, Skaggs WE, Buonocore MH, Rapp PR, & Barnes CA (2008). Age-related regional network of magnetic resonance imaging gray matter in the rhesus macaque. The Journal of Neuroscience, 28(11), 2710–2718. 10.1523/JNEUROSCI.1852-07.2008 - DOI - PMC - PubMed
-
- Bachevalier J, Landis LS, Walker LC, Brickson M, Mishkin M, Price DL, & Cork LC (1991). Aged monkeys exhibit behavioral deficits indicative of widespread cerebral dysfunction. Neurobiology of Aging, 12(2), 99–111. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

