Computationally-Assisted Discovery and Assignment of a New Class of 6/6/5/5 Fused-Ring Diterpene Acting as Pregnane X Receptor Ligands from Isodon serra
- PMID: 39315450
- PMCID: PMC12582125
- DOI: 10.1021/acs.jnatprod.4c00759
Computationally-Assisted Discovery and Assignment of a New Class of 6/6/5/5 Fused-Ring Diterpene Acting as Pregnane X Receptor Ligands from Isodon serra
Abstract
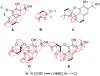
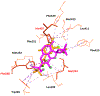
We report here the orchestration of molecular ion networking (MoIN) and a set of computationally assisted structural elucidation approaches in the discovery and assignment of a new class of rearranged 4,5-seco-abietane diterpenoids including serra A (1), which possesses an unusual 6/6/5/5 fused-ring skeleton system, together with two previously unreported diterpenoids serras B-C (2-3) and five known compounds were isolated from Isodon serra (I. serra). The structures were elucidated by spectroscopic analysis in conjunction with computationally assisted structure elucidation tools. In silico, serras A-C (1-3) bind well to PXR, suggesting their potential role in reducing inflammation. The results of serra A (1) with hPXR demonstrated agonist activity with an EC50 value of 15 μM. Serra A (1), graciliflorin F (4), gerardianin C (5), 11,12,15-trihydroxy-8,11,13-abietatrien-7-one (6), rabdosin D (7), and 15-hydroxysalprionin (8) exhibited promising anti-inflammatory activities in lipopolysaccharide (LPS)-induced RAW 267.4 cells, and their inhibition rates on NO production were more than 65% at 10 μM.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

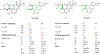

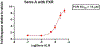
References
-
- Dvorak Z; Kopp F; Costello CM; Kemp JS; Li H; Vrzalova A; Stepankova M; Bartonkova I; Jiskrova E; Poulikova K; Vyhlidalova B; Nordstroem LU; Karunaratne CV; Ranhotra HS; Mun KS; Naren AP; Murray IA; Perdew GH; Brtko J; Toporova L; Schon A; Wallace BD; Walton WG; Redinbo MR; Sun K; Beck A; Kortagere S; Neary MC; Chandran A; Vishveshwara S; Cavalluzzi MM; Lentini G; Cui JY; Gu H; March JC; Chatterjee S; Matson A; Wright D; Flannigan KL; Hirota SA; Sartor RB; Mani S EMBO Mol. Med 2020, 12, No. e11621. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

