Assessing signs of central sensitization: A critical review of physiological measures in experimentally induced secondary hyperalgesia
- PMID: 39315535
- PMCID: PMC11754940
- DOI: 10.1002/ejp.4733
Assessing signs of central sensitization: A critical review of physiological measures in experimentally induced secondary hyperalgesia
Abstract
Background and objectives: Central sensitization (CS) is believed to play a role in many chronic pain conditions. Direct non-invasive recording from single nociceptive neurons is not feasible in humans, complicating CS establishment. This review discusses how secondary hyperalgesia (SHA), considered a manifestation of CS, affects physiological measures in healthy individuals and if these measures could indicate CS. It addresses controversies about heat sensitivity changes, the role of tactile afferents in mechanical hypersensitivity and detecting SHA through electrical stimuli. Additionally, it reviews the potential of neurophysiological measures to indicate CS presence.
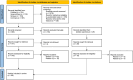
Databases and data treatment: Four databases, PubMed, ScienceDirect, Scopus and Cochrane Library, were searched using terms linked to 'hyperalgesia'. The search was limited to research articles in English conducted in humans until 2023.
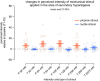
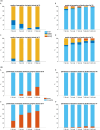
Results: Evidence for heat hyperalgesia in the SHA area is sparse and seems to depend on the experimental method used. Minimal or no involvement of tactile afferents in SHA was found. At the spinal level, the threshold of the nociceptive withdrawal reflex (RIII) is consistently reduced during experimentally induced SHA. The RIII area and the spinal somatosensory potential (N13-SEP) amplitude are modulated only with long-lasting nociceptive input. At the brain level, pinprick-evoked potentials within the SHA area are increased.
Conclusions: Mechanical pinprick hyperalgesia is the most reliable behavioural readout for SHA, while the RIII threshold is the most sensitive neurophysiological readout. Due to scarce data on reliability, sensitivity and specificity, none of the revised neurophysiological methods is currently suitable for CS identification at the individual level.
Significance: Gathering evidence for CS in humans is a crucial research focus, especially with the increasing interest in concepts such as 'central sensitization-like pain' or 'nociplastic pain'. This review clarifies which readouts, among the different behavioural and neurophysiological proxies tested in experimental settings, can be used to infer the presence of CS in humans.
© 2024 The Author(s). European Journal of Pain published by John Wiley & Sons Ltd on behalf of European Pain Federation ‐ EFIC ®.
Figures


Similar articles
-
How different experimental models of secondary hyperalgesia change the nociceptive flexion reflex.Clin Neurophysiol. 2021 Dec;132(12):2989-2995. doi: 10.1016/j.clinph.2021.08.018. Epub 2021 Oct 5. Clin Neurophysiol. 2021. PMID: 34715423
-
Modulation of the spinal N13 SEP component by high- and low-frequency electrical stimulation. Experimental pain models matter.Clin Neurophysiol. 2023 Dec;156:28-37. doi: 10.1016/j.clinph.2023.08.022. Epub 2023 Oct 6. Clin Neurophysiol. 2023. PMID: 37856896
-
Healthy women show more experimentally induced central sensitization compared with men.Pain. 2024 Jun 1;165(6):1413-1424. doi: 10.1097/j.pain.0000000000003144. Epub 2024 Jan 11. Pain. 2024. PMID: 38231588 Free PMC article.
-
Post-treatment lyme syndrome and central sensitization.J Neuropsychiatry Clin Neurosci. 2013 Summer;25(3):176-86. doi: 10.1176/appi.neuropsych.12090223. J Neuropsychiatry Clin Neurosci. 2013. PMID: 24026711 Review.
-
Clinical neurophysiology of neuropathic pain.Int Rev Neurobiol. 2024;179:125-154. doi: 10.1016/bs.irn.2024.10.005. Epub 2024 Nov 4. Int Rev Neurobiol. 2024. PMID: 39580211 Review.
Cited by
-
The effect of psychological manipulations on the development of secondary hyperalgesia: a critical review.Pain Rep. 2025 May 27;10(4):e1291. doi: 10.1097/PR9.0000000000001291. eCollection 2025 Aug. Pain Rep. 2025. PMID: 40444022 Free PMC article. Review.
-
Injury-Driven Structural and Molecular Modifications in Nociceptors.Biology (Basel). 2025 Jun 29;14(7):788. doi: 10.3390/biology14070788. Biology (Basel). 2025. PMID: 40723348 Free PMC article. Review.
-
Understanding neuropathic pain: the role of neurophysiological tests in unveiling underlying mechanisms.J Anesth Analg Crit Care. 2024 Nov 18;4(1):77. doi: 10.1186/s44158-024-00212-z. J Anesth Analg Crit Care. 2024. PMID: 39558394 Free PMC article. Review.
References
-
- Ali, Z. , Meyer, R. A. , & Campbell, J. N. (1996). Secondary hyperalgesia to mechanical but not heat stimuli following a capsaicin injection in hairy skin. Pain, 68, 401–411. - PubMed
-
- Andersen, O. K. , Felsby, S. , Nicolaisen, L. , Bjerring, P. , Jensen, T. S. , & Arendt‐Nielsen, L. (1996). The effect of ketamine on stimulation of primary and secondary hyperalgesic areas induced by capsaicin—a double‐blind, placebo‐controlled, human experimental study. Pain, 66, 51–62. - PubMed
-
- Andersen, O. K. , Gracely, R. H. , & Arednt‐Nielsen, L. (1995). Facilitation of the human nociceptive reflex by stimulation of Aβ‐fibres in a secondary hyperalgesic area sustained by nociceptive input from the primary hyperalgesic area. Acta Physiologica Scandinavica, 155, 87–97. - PubMed
-
- Andersen, O. K. , Spaich, E. G. , Madeleine, P. , & Arendt‐Nielsen, L. (2005). Gradual enlargement of human withdrawal reflex receptive fields following repetitive painful stimulation. Brain Research, 1042, 194–204. - PubMed
-
- Andrews, K. , Baranowski, A. , & Kinnman, E. (1999). Sensory threshold changes without initial pain or alterations in cutaneous blood flow, in the area of secondary hyperalgesia caused by topical application of capsaicin in humans. Neuroscience Letters, 266, 45–48. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

