Role of DNA Double-Strand Break Formation in Gyrase Inhibitor-Mediated Killing of Nonreplicating Persistent Mycobacterium tuberculosis in Caseum
- PMID: 39315541
- PMCID: PMC11474946
- DOI: 10.1021/acsinfecdis.4c00499
Role of DNA Double-Strand Break Formation in Gyrase Inhibitor-Mediated Killing of Nonreplicating Persistent Mycobacterium tuberculosis in Caseum
Abstract
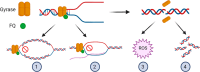
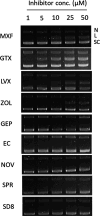
Tuberculosis is the leading cause of mortality by infectious agents worldwide. The necrotic debris, known as caseum, which accumulates in the center of pulmonary lesions and cavities is home to nonreplicating drug-tolerant Mycobacterium tuberculosis that presents a significant hurdle to achieving a fast and durable cure. Fluoroquinolones such as moxifloxacin are highly effective at killing this nonreplicating persistent bacterial population and boosting TB lesion sterilization. Fluoroquinolones target bacterial DNA gyrase, which catalyzes the negative supercoiling of DNA and relaxes supercoils ahead of replication forks. In this study, we investigated the potency of several other classes of gyrase inhibitors against M. tuberculosis in different states of replication. In contrast to fluoroquinolones, many other gyrase inhibitors kill only replicating bacterial cultures but produce negligible cidal activity against M. tuberculosis in ex vivo rabbit caseum. We demonstrate that while these inhibitors are capable of inhibiting M. tuberculosis gyrase DNA supercoiling activity, fluoroquinolones are unique in their ability to cleave double-stranded DNA at low micromolar concentrations. We hypothesize that double-strand break formation is an important driver of gyrase inhibitor-mediated bactericidal potency against nonreplicating persistent M. tuberculosis populations in the host. This study provides general insight into the lesion sterilization potential of different gyrase inhibitor classes and informs the development of more effective chemotherapeutic options against persistent mycobacterial infections.
Keywords: Mycobacterium tuberculosis; caseum; double-strand break; fluoroquinolones; nonreplicating persistence.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



Similar articles
-
Mycobacterium tuberculosis DNA gyrase as a target for drug discovery.Infect Disord Drug Targets. 2007 Jun;7(2):159-68. doi: 10.2174/187152607781001763. Infect Disord Drug Targets. 2007. PMID: 17970226 Review.
-
Mechanism of Action of Mycobacterium tuberculosis Gyrase Inhibitors: A Novel Class of Gyrase Poisons.ACS Infect Dis. 2018 Aug 10;4(8):1211-1222. doi: 10.1021/acsinfecdis.8b00035. Epub 2018 May 17. ACS Infect Dis. 2018. PMID: 29746087 Free PMC article.
-
Extreme Drug Tolerance of Mycobacterium tuberculosis in Caseum.Antimicrob Agents Chemother. 2018 Jan 25;62(2):e02266-17. doi: 10.1128/AAC.02266-17. Print 2018 Feb. Antimicrob Agents Chemother. 2018. PMID: 29203492 Free PMC article.
-
Molecular basis for the differential quinolone susceptibility of mycobacterial DNA gyrase.Antimicrob Agents Chemother. 2014;58(4):2013-20. doi: 10.1128/AAC.01958-13. Epub 2014 Jan 13. Antimicrob Agents Chemother. 2014. PMID: 24419347 Free PMC article.
-
Targeting DNA Gyrase to Combat Mycobacterium tuberculosis: An Update.Curr Top Med Chem. 2019;19(8):579-593. doi: 10.2174/1568026619666190304130218. Curr Top Med Chem. 2019. PMID: 30834837 Review.
Cited by
-
A Physiologically Relevant In Vitro Model of Nonreplicating Persistent Mycobacterium tuberculosis in Caseum.Curr Protoc. 2025 Mar;5(3):e70118. doi: 10.1002/cpz1.70118. Curr Protoc. 2025. PMID: 40056090 Free PMC article.
-
Targeting de novo purine biosynthesis for tuberculosis treatment.Nature. 2025 Aug;644(8075):214-220. doi: 10.1038/s41586-025-09177-7. Epub 2025 Jun 18. Nature. 2025. PMID: 40533558 Free PMC article.
-
Antibacterial carbon dots.Mater Today Bio. 2024 Dec 5;30:101383. doi: 10.1016/j.mtbio.2024.101383. eCollection 2025 Feb. Mater Today Bio. 2024. PMID: 39811607 Free PMC article. Review.
References
-
- Blanc L.; Sarathy J. P.; Cabrera N. A.; O’Brien P.; Dias-Freedman I.; Mina M.; Sacchettini J.; Savic R. M.; Gengenbacher M.; Podell B. K.; Prideaux B.; Ioerger T.; Dick T.; Dartois V. Impact of immunopathology on the antituberculous activity of pyrazinamide. J. Exp. Med. 2018, 215 (8), 1975–1986. 10.1084/jem.20180518. - DOI - PMC - PubMed
-
- Lin P. L.; Ford C. B.; Coleman M. T.; Myers A. J.; Gawande R.; Ioerger T.; Sacchettini J.; Fortune S. M.; Flynn J. L. Sterilization of granulomas is common in active and latent tuberculosis despite within-host variability in bacterial killing. Nat. Med. 2014, 20 (1), 75–79. 10.1038/nm.3412. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

