The efficacy of gemcitabine and docetaxel chemotherapy for the treatment of relapsed and refractory osteosarcoma: A systematic review and pre-clinical study
- PMID: 39315544
- PMCID: PMC11420655
- DOI: 10.1002/cam4.70248
The efficacy of gemcitabine and docetaxel chemotherapy for the treatment of relapsed and refractory osteosarcoma: A systematic review and pre-clinical study
Abstract
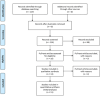
Introduction: Osteosarcoma is the most common primary malignancy of the bone. There is a lack of effective treatments for patients who experience relapsed osteosarcoma. One treatment for relapsed patients is gemcitabine and docetaxel combination chemotherapy (GEMDOX). This systematic review aimed to establish the efficacy of this chemotherapy regimen, as well as identify the common severe toxicities that are associated with it. Resistant osteosarcoma cell lines developed from MG-63 and HOS-143B were used to represent relapsed osteosarcoma patients in a pre-clinical study.
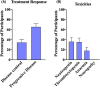
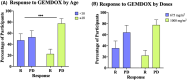
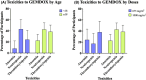
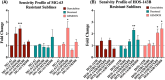
Results: We identified 11 retrospective and Phase II studies that were suitable for inclusion in our review. 10.65% of patients had a response to gemcitabine and docetaxel combination therapy and the disease control rate was 35% (n = 197). 36%, 35.3% and 18.04% of patients experienced grade 3 or 4 neutropenia, thrombocytopenia and anaemia respectively (n = 133). Male patients (X2 = 9.14, p < 0.05) and those below the age of 18 (X 2 = 10.94, p < 0.05) responded better to GEMDOX treatment than females and patients older than 18 years. The resistant osteosarcoma cell lines remained sensitive to either single-agent gemcitabine, docetaxel, and the combination of both. Cisplatin-resistant models (MG-63/CISR8 & HOS-143B/CISR8) were the most responsive to GEMDOX treatment compared to doxorubicin, methotrexate, and triple-combination resistant models.
Conclusion: GEMDOX treatment has potential efficacy in relapsed osteosarcoma patients especially those with cisplatin resistance. To directly compare the efficacy of GEMDOX therapy against other therapies randomised phase III clinical trials with adequate patient follow up must be performed to improve treatment options for osteosarcoma.
Keywords: docetaxel; gemcitabine; relapsed osteosarcoma.
© 2024 The Author(s). Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Efficacy and safety of gemcitabine-docetaxel combination therapy for recurrent or refractory high-grade osteosarcoma in China: a retrospective study of 18 patients.Jpn J Clin Oncol. 2012 May;42(5):427-31. doi: 10.1093/jjco/hys030. Epub 2012 Mar 26. Jpn J Clin Oncol. 2012. PMID: 22450929
-
Comparison of pirarubicin-based versus gemcitabine-docetaxel chemotherapy for relapsed and refractory osteosarcoma: a single institution experience.Int J Clin Oncol. 2013 Jun;18(3):498-505. doi: 10.1007/s10147-012-0409-5. Epub 2012 Apr 26. Int J Clin Oncol. 2013. PMID: 22534798
-
Gemcitabine and docetaxel combination chemotherapy for advanced bone and soft tissue sarcomas: protocol for an open-label, non-randomised, Phase 2 study.BMC Cancer. 2019 Jul 23;19(1):725. doi: 10.1186/s12885-019-5923-7. BMC Cancer. 2019. PMID: 31337342 Free PMC article.
-
The role of chemotherapy for metastatic, relapsed and refractory osteosarcoma.Paediatr Drugs. 2014 Dec;16(6):503-12. doi: 10.1007/s40272-014-0095-z. Paediatr Drugs. 2014. PMID: 25392156 Review.
-
Docetaxel/gemcitabine: salvage chemotherapy in anthracycline-pretreated patients with advanced breast cancer.Oncology (Williston Park). 2001 Feb;15(2 Suppl 3):18-24. Oncology (Williston Park). 2001. PMID: 11252884 Review.
Cited by
-
Advancements in Osteosarcoma Therapy: Overcoming Chemotherapy Resistance and Exploring Novel Pharmacological Strategies.Pharmaceuticals (Basel). 2025 Apr 3;18(4):520. doi: 10.3390/ph18040520. Pharmaceuticals (Basel). 2025. PMID: 40283955 Free PMC article. Review.
-
S100 A16 promotes the progression of osteosarcoma by activating the PI3 K/AKT signaling pathway through ANXA2.Sci Rep. 2025 Jun 6;15(1):19962. doi: 10.1038/s41598-025-05293-6. Sci Rep. 2025. PMID: 40481236 Free PMC article.
-
Single-cell analysis links DCUN1D5 to immune remodeling and cisplatin resistance in recurrent osteosarcoma.Commun Biol. 2025 Jul 8;8(1):1019. doi: 10.1038/s42003-025-08409-w. Commun Biol. 2025. PMID: 40629059 Free PMC article.
-
Circular RNAs modulate cancer drug resistance: advances and challenges.Cancer Drug Resist. 2025 Mar 28;8:17. doi: 10.20517/cdr.2024.195. eCollection 2025. Cancer Drug Resist. 2025. PMID: 40201313 Free PMC article.
References
-
- Marina NM, Smeland S, Bielack SS, et al. Comparison of MAPIE versus MAP in patients with a poor response to preoperative chemotherapy for newly diagnosed high‐grade osteosarcoma (EURAMOS‐1): an open‐label, international, randomised controlled trial. Lancet Oncol. 2016;17(10):1396‐1408. doi:10.1016/S1470-2045(16)30214-5 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

