Pyruvate metabolism dictates fibroblast sensitivity to GLS1 inhibition during fibrogenesis
- PMID: 39315548
- PMCID: PMC11457851
- DOI: 10.1172/jci.insight.178453
Pyruvate metabolism dictates fibroblast sensitivity to GLS1 inhibition during fibrogenesis
Abstract
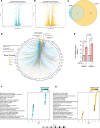
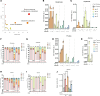
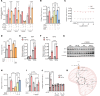
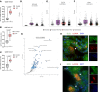
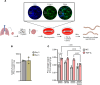
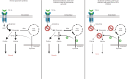
Fibrosis is a chronic disease characterized by excessive extracellular matrix production, which leads to disruption of organ function. Fibroblasts are key effector cells of this process, responding chiefly to the pleiotropic cytokine transforming growth factor-β1 (TGF-β1), which promotes fibroblast to myofibroblast differentiation. We found that extracellular nutrient availability profoundly influenced the TGF-β1 transcriptome of primary human lung fibroblasts and that biosynthesis of amino acids emerged as a top enriched TGF-β1 transcriptional module. We subsequently uncovered a key role for pyruvate in influencing glutaminase (GLS1) inhibition during TGF-β1-induced fibrogenesis. In pyruvate-replete conditions, GLS1 inhibition was ineffective in blocking TGF-β1-induced fibrogenesis, as pyruvate can be used as the substrate for glutamate and alanine production via glutamate dehydrogenase (GDH) and glutamic-pyruvic transaminase 2 (GPT2), respectively. We further show that dual targeting of either GPT2 or GDH in combination with GLS1 inhibition was required to fully block TGF-β1-induced collagen synthesis. These findings embolden a therapeutic strategy aimed at additional targeting of mitochondrial pyruvate metabolism in the presence of a glutaminolysis inhibitor to interfere with the pathological deposition of collagen in the setting of pulmonary fibrosis and potentially other fibrotic conditions.
Keywords: Cell biology; Collagens; Fibrosis; Glucose metabolism; Metabolism.
Conflict of interest statement
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

