Extracellular vesicles derived from melanoma cells induce carcinoma-associated fibroblasts via miR-92b-3p mediated downregulation of PTEN
- PMID: 39315679
- PMCID: PMC11420832
- DOI: 10.1002/jev2.12509
Extracellular vesicles derived from melanoma cells induce carcinoma-associated fibroblasts via miR-92b-3p mediated downregulation of PTEN
Abstract
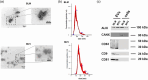
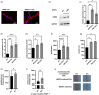
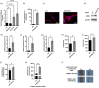
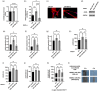
In melanoma, carcinoma-associated fibroblasts (CAFs) are important cellular components in the tumour microenvironment due to their potential to promote tumour growth and metastatic spread of malignant cells. Melanoma cells have the ability to affect non-tumour cells in the microenvironment by releasing extracellular vesicles (EVs). The mechanisms responsible for reprogramming normal dermal fibroblasts (NHDFs) into CAFs remain incompletely understood. However, it is likely thought to be mediated by melanoma-specific miRNAs, which are transported by EVs derived from melanoma cells. Therefore, we wondered if one of the most enriched miRNAs in EVs secreted by melanoma cells, miR-92b-3p, is involved in the conversion of normal fibroblasts into CAFs. We observed that melanoma cell-derived EVs indeed delivered miR-92b-3p into NHDFs and that its accumulation correlated with CAF formation, as demonstrated by enhanced expression of CAF marker genes and increased proliferation and migration. Overexpression of miR-92b-3p in NHDFs revealed similar results, while EVs deficient of miR-92b-3p did not induce a CAF phenotype. As a target we identified PTEN, whose repression led to increased expression of CAF markers. We thus provide a novel pathway of intercellular communication by which melanoma cells control the transformation of CAFs by virtue of EV-transported miRNAs.
Keywords: carcinoma‐associated fibroblasts; extracellular vesicles; melanoma; miRNA.
© 2024 The Authors. Journal of Extracellular Vesicles published by Wiley Periodicals, LLC on behalf of the International Society for Extracellular Vesicles.
Conflict of interest statement
The authors declare they have no conflict of interest.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

