Giant Thermosalient Effect in a Molecular Single Crystal: Dynamic Transformations and Mechanistic Insights
- PMID: 39315726
- PMCID: PMC11467902
- DOI: 10.1021/jacs.4c09222
Giant Thermosalient Effect in a Molecular Single Crystal: Dynamic Transformations and Mechanistic Insights
Abstract
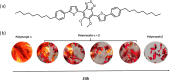
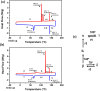
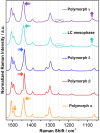
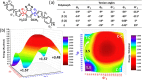
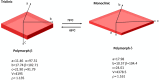
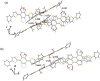
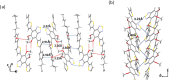
The exploration of mechanical motion in molecular crystals under external stimuli is of great interest because of its potential applications in diverse fields, such as electronics, actuation, or sensing. Understanding the underlying processes, including phase transitions and structural changes, is crucial for exploiting the dynamic nature of these crystals. Here, we present a novel organic compound, PT-BTD, consisting of five interconnected aromatic units and two peripheral alkyl chains, which forms crystals that undergo a drastic anisotropic expansion (33% in the length of one of its dimensions) upon thermal stimulation, resulting in a pronounced deformation of their crystal shape. Remarkably, the transformation occurs while maintaining the single-crystal nature, which has allowed us to follow the crystal-to-crystal transformation by single-crystal analysis of the initial and expanded polymorphs, providing valuable insights into the underlying mechanisms of this unique thermosalient behavior. At the molecular level, this transformation is associated with subtle, coordinated conformational changes, including slight rotations of the five interconnected aromatic units in its structure and increased dynamism in one of its peripheral alkyl chains as the temperature rises, leading to the displacement of the molecules. In situ polarized optical microscopy reveals that this transformation occurs as a rapidly advancing front, indicative of a martensitic phase transition. The results of this study highlight the crucial role of a soft and flexible structural configuration combined with a highly compact but loosely bound supramolecular structure in the design of thermoelastic materials.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
-
- Urban M. W.Handbook of Stimuli-Responsive Materials; Wiley-VCH, 2011.
-
- Stuart M. A. C.; Huck W. T. S.; Genzer J.; Müller M.; Ober C.; Stamm M.; Sukhorukov G. B.; Szleifer I.; Tsukruk V. V.; Urban M.; Winnik F.; Zauscher S.; Luzinov I.; Minko S. Emerging Applications of Stimuli-Responsive Polymer Materials. Nat. Mater. 2010, 9 (2), 101–113. 10.1038/nmat2614. - DOI - PubMed
-
- Zheng R.; Wei Y.; Zhang Z.-C.; Wang Z.-Y.; Ma L.-L.; Wang Y.; Huang L.; Lu Y.-Q. Stimuli-Responsive Active Materials for Dynamic Control of Light Field. Responsive Mater. 2023, 1, e2023001710.1002/rpm.20230017. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

