Time to use the right classification to predict the severity of checkpoint inhibitor-induced liver injury, as assessed for causality using the updated RUCAM
- PMID: 39315730
- PMCID: PMC11599793
- DOI: 10.1111/apt.18276
Time to use the right classification to predict the severity of checkpoint inhibitor-induced liver injury, as assessed for causality using the updated RUCAM
Abstract
Background and aims: While immune checkpoint inhibitors (ICIs) are revolutionising cancer therapy, checkpoint inhibitor-induced liver injury is a significant immune-related side effect of this immunotherapy. This study focuses on the severity classifications and characteristics of patients with checkpoint inhibitor-induced hepatitis.
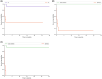
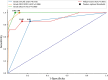
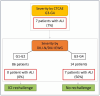
Methods: A retrospective analysis of patients with severe Checkpoint Inhibitor-induced hepatitis grade 3 and 4 according to the recommended Common Terminology Criteria for Adverse Events (CTCAE) classification was conducted. Data on clinicobiological characteristics, treatment and outcomes were collected from 3 university hospitals, and causality was assessed by using the updated Roussel Uclaf Causality Assessment Method. The severity of hepatitis was assessed using the Model for End-stage Liver Disease score, the Drug-Induced Liver Injury Network, and the Drug-Induced Liver Injury International Expert Working Group classifications.
Results: We retrospectively included 100 patients presenting various hepatitis patterns with a median time to onset of 20 days after checkpoint inhibitors. Severity grading varied significantly among the classifications used. A lower incidence of severe cases was observed when using the Drug-Induced Liver Injury classifications instead of the recommended CCTCAE classification, and this was correlated with outcomes.
Conclusions: This retrospective study challenges the efficacy of the CTCAE classification in defining the severity of Checkpoint Inhibitor-induced hepatitis and suggests that the traditional hepatology-focused scores may be more relevant. The CTCAE classification is inconsistent and gives equal weight to jaundice and elevated transaminases, which leads to steroid overtreatment and limits the rechallenge of ICIs.
© 2024 The Author(s). Alimentary Pharmacology & Therapeutics published by John Wiley & Sons Ltd.
Figures

References
-
- La‐Beck NM, Jean GW, Huynh C, Alzghari SK, Lowe DB. Immune checkpoint inhibitors: new insights and current place in cancer therapy. Pharmacotherapy. 2015;35(10):963–976. - PubMed
-
- Schmid P, Cortes J, Dent R, Pusztai L, McArthur H, Kümmel S, et al. Event‐free survival with Pembrolizumab in early triple‐negative breast cancer. N Engl J Med. 2022;386(6):556–567. - PubMed
-
- Fife BT, Bluestone JA. Control of peripheral T‐cell tolerance and autoimmunity via the CTLA‐4 and PD‐1 pathways. Immunol Rev. 2008;224:166–182. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
