Time-resolved proximity proteomics uncovers a membrane tension-sensitive caveolin-1 interactome at the rear of migrating cells
- PMID: 39315773
- PMCID: PMC11509677
- DOI: 10.7554/eLife.85601
Time-resolved proximity proteomics uncovers a membrane tension-sensitive caveolin-1 interactome at the rear of migrating cells
Abstract
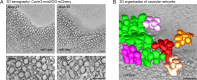
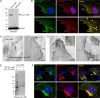
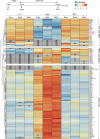
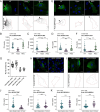
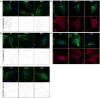
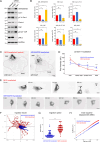
Caveolae are small membrane pits with fundamental roles in mechanotransduction. Several studies have shown that caveolae flatten out in response to increased membrane tension, thereby acting as a mechanosensitive membrane reservoir that buffers acute mechanical stress. Caveolae have also been implicated in the control of RhoA/ROCK-mediated actomyosin contractility at the rear of migrating cells. However, how membrane tension controls the organisation of caveolae and their role in mechanotransduction remains unclear. To address this, we systematically quantified protein-protein interactions of caveolin-1 in migrating RPE1 cells at steady state and in response to an acute increase in membrane tension using biotin-based proximity labelling and quantitative mass spectrometry. Our data show that caveolae are highly enriched at the rear of migrating RPE1 cells and that membrane tension rapidly and reversibly disrupts the caveolar protein coat. Membrane tension also detaches caveolin-1 from focal adhesion proteins and several mechanosensitive regulators of cortical actin including filamins and cortactin. In addition, we present evidence that ROCK and the RhoGAP ARHGAP29 associate with caveolin-1 in a manner dependent on membrane tension, with ARHGAP29 influencing caveolin-1 Y14 phosphorylation, caveolae rear localisation, and RPE1 cell migration. Taken together, our work uncovers a membrane tension-sensitive coupling between caveolae and the rear-localised F-actin cytoskeleton. This provides a framework for dissecting the molecular mechanisms underlying caveolae-regulated mechanotransduction pathways.
Keywords: APEX2; caveolae; caveolin-1; cavin; cell biology; cell migration; human; mechanobiology; membrane tension; proximity proteomics.
© 2024, Martin, Girardello et al.
Conflict of interest statement
EM, RG, GD, AL No competing interests declared
Figures









Update of
- doi: 10.1101/2022.12.13.520222
References
-
- Cheng JPX, Mendoza-Topaz C, Howard G, Chadwick J, Shvets E, Cowburn AS, Dunmore BJ, Crosby A, Morrell NW, Nichols BJ. Caveolae protect endothelial cells from membrane rupture during increased cardiac output. The Journal of Cell Biology. 2015;211:53–61. doi: 10.1083/jcb.201504042. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

