Hydroxychloroquine monotherapy in sarcoidosis: Indications, efficacy, and side effects
- PMID: 39315981
- PMCID: PMC11472668
- DOI: 10.36141/svdld.v41i3.15445
Hydroxychloroquine monotherapy in sarcoidosis: Indications, efficacy, and side effects
Abstract
Background and aim: In sarcoidosis which is not threatening vital internal organs such as heart, central nervous system or lungs immunomodulatory treatment, such as hydroxychloroquine can be considered. Despite its common use, limited data are available regarding effectiveness and side effects as monotherapy in sarcoidosis. Recommendations on its usage are based on expert opinion as literature is scarce.
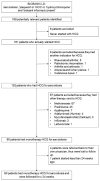
Methods: This retrospective study examines real-world data about the indications, effectivity and side effects of hydroxychloroquine monotherapy in sarcoidosis patients with no damage to the vital internal organs. Successful treatment was defined as continuation after 24 weeks without step up therapy or worsening of symptoms.
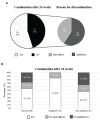
Results: Sixty patients were eligible for the study. Starting dose was 400mg/day, lowered to 200mg/day after 3 months in most patients. The predominant treatment indications were musculoskeletal 45 (75%) and cutaneous involvement 13 (22%). Thirty-three patients (55%) continued treatment after 24 weeks. Twenty-four patients (40%) mentioned side effects, mainly gastrointestinal, leading to treatment discontinuation in eleven patients (18%). No severe side effects were seen. Continuation after 24 weeks was significantly higher in patients with cutaneous involvement compared to other indications 85% vs 47% respectively (p =0.02).
Conclusions: Treatment with hydroxychloroquine monotherapy was satisfactory in 55% of patients, especially for cutaneous involvement. However, it poses considerable non-severe side-effects.
Conflict of interest statement
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript. The authors have no relevant financial or non-financial interests to disclose. The study was approved approved by the local ethics committee (MEC-U) under study number R05-08A. All patients gave written informed consent.
Figures
References
-
- Grunewald J, Grutters JC, Arkema E V, Saketkoo LA, Moller DR, Müller-Quernheim J. Sarcoidosis. Nat Rev Dis Primers [Internet] 2019 Dec 1 [cited 2021 Dec 19];5(1) Available from: https://pubmed.ncbi.nlm.nih.gov/31273209/ - PubMed
-
- Kahlmann V, Moor CC, Veltkamp M, Wijsenbeek MS. Patient reported side-effects of prednisone and methotrexate in a real-world sarcoidosis population. Chron Respir Dis [Internet] 2021 [cited 2023 Apr 2];18:1–4. Available from: /pmc/articles/PMC8477709/ - PMC - PubMed
-
- Thillai M, Atkins CP, Crawshaw A, et al. BTS Clinical Statement on pulmonary sarcoidosis. Thorax. 2021 Jan 1;76(1):4–20. - PubMed
-
- Baughman RP, Valeyre D, Korsten P, et al. ERS clinical practice guidelines on treatment of sarcoidosis. European Respiratory Journal [Internet] 2021 [cited 2023 Mar 4];58(21) doi:10.1183/13993003.04079-2020. - PubMed
-
- Baughman RP, Judson MA, Wells A. The indications for the treatment of sarcoidosis: Wells Law. Sarcoidosis, Vasculitis, and Diffuse Lung Diseases [Internet] 2017 [cited 2022 Feb 24];34(4):280. Available from: /pmc/articles/PMC7170078/ - PMC - PubMed
LinkOut - more resources
Full Text Sources
