Transport of β-amyloid from brain to eye causes retinal degeneration in Alzheimer's disease
- PMID: 39316084
- PMCID: PMC11448872
- DOI: 10.1084/jem.20240386
Transport of β-amyloid from brain to eye causes retinal degeneration in Alzheimer's disease
Abstract
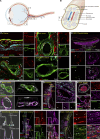
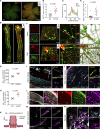
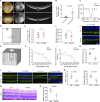
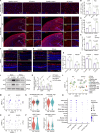
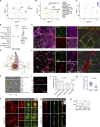
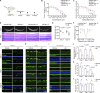
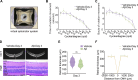
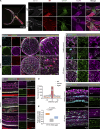
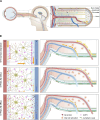
The eye is closely connected to the brain, providing a unique window to detect pathological changes in the brain. In this study, we discovered β-amyloid (Aβ) deposits along the ocular glymphatic system in patients with Alzheimer's disease (AD) and 5×FAD transgenic mouse model. Interestingly, Aβ from the brain can flow into the eyes along the optic nerve through cerebrospinal fluid (CSF), causing retinal degeneration. Aβ is mainly observed in the optic nerve sheath, the neural axon, and the perivascular space, which might represent the critical steps of the Aβ transportation from the brain to the eyes. Aquaporin-4 facilitates the influx of Aβ in brain-eye transport and out-excretion of the retina, and its absence or loss of polarity exacerbates brain-derived Aβ induced damage and visual impairment. These results revealed brain-to-eye Aβ transport as a major contributor to AD retinopathy, highlighting a new therapeutic avenue in ocular and neurodegenerative disease.
© 2024 Cao et al.
Conflict of interest statement
Disclosures: M. Nedergaard reported personal fees from CTN2 outside the submitted work and is a paid consultant for CNS2 for unrelated work. No other disclosures were reported.
Figures






References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

