Inflammasome activation aggravates choroidal neovascularization
- PMID: 39316206
- PMCID: PMC11563918
- DOI: 10.1007/s10456-024-09949-1
Inflammasome activation aggravates choroidal neovascularization
Abstract
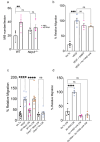
Inflammasome activation is implicated in diseases of aberrant angiogenesis such as age-related macular degeneration (AMD), though its precise role in choroidal neovascularization (CNV), a characteristic pathology of advanced AMD, is ill-defined. Reports on inhibition of inflammasome constituents on CNV are variable and the precise role of inflammasome in mediating pathological angiogenesis is unclear. Historically, subretinal injection of inflammasome agonists alone has been used to investigate retinal pigmented epithelium (RPE) degeneration, while the laser photocoagulation model has been used to study pathological angiogenesis in a model of CNV. Here, we report that the simultaneous introduction of any of several disease-relevant inflammasome agonists (Alu or B2 RNA, Alu cDNA, or oligomerized amyloid β (1-40)) exacerbates laser-induced CNV. These activities were diminished or abrogated by genetic or pharmacological targeting of inflammasome signaling constituents including P2rx7, Nlrp3, caspase-1, caspase-11, and Myd88, as well as in myeloid-specific caspase-1 knockout mice. Alu RNA treatment induced inflammasome activation in macrophages within the CNV lesion, and increased accumulation of macrophages in an inflammasome-dependent manner. Finally, IL-1β neutralization prevented inflammasome agonist-induced chemotaxis, macrophage trafficking, and angiogenesis. Collectively, these observations support a model wherein inflammasome stimulation promotes and exacerbates CNV and may be a therapeutic target for diseases of angiogenesis such as neovascular AMD.
Keywords: Age-related macular degeneration; Choroidal neovascularization; Inflammasome; Interleukin-1beta; Macrophage; Myd88.
© 2024. The Author(s).
Conflict of interest statement
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

