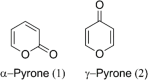
Natural products with γ-pyrone scaffold from Streptomyces
- PMID: 39316232
- PMCID: PMC11422467
- DOI: 10.1007/s00253-024-13296-y
Natural products with γ-pyrone scaffold from Streptomyces
Abstract
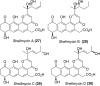
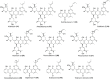
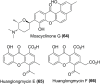
The Streptomyces sp. is considered the vast reservoir of bioactive natural products belonging to different classes like polyketides, terpenoids, lanthipeptides, and non-ribosomal peptides to name a few. The ubiquitous distribution of the genus makes them capable of producing distinct compounds. Many of those compounds contain a unique γ-pyrone with various chemical structures and exhibit different bioactivities. One such class, nitrophenyl-γ-pyrone, constitutes different bioactive compounds isolated from Streptomyces sp. from different sources ranging from soil to marine environments. In addition, such compounds have antinematodal, cytotoxicity activities, and inhibition of adipogenesis. These compounds include aureothin (3), spectinabilin (7), and their derivatives. Moreover, there are other compounds like actinopyrones (11-16), benwamycins (22-23), and peucemycin and its derivatives (24-26) that also have antibacterial and anticancer activities. The other group classified as anthra-γ-pyrone has various bioactive natural products. For instance, tetrahydroanthra-γ-pyrone, shellmycin A-D (27-30) possess antibacterial as well as anticancer activities. In addition, the pluramycin family compounds belonging to anthra-γ-pyrone group also possess cytotoxic activity, for instance, kidamycin (31), rubiflavin, and their derivatives (33-37). Xanthones are another important group of natural products that also contain γ-pyrone ring producing different bioactivities. Albofungin (42) and its derivatives (43-46) belong to subgroup polycyclic tetrahydro xanthones that possess antibacterial, anticancer, and antibiofilm, antimacrofouling activities. Similarly, other compounds, belonging to this subgroup, exhibit different bioactivities like antifungal, antimalarial, and antibacterial activities and block transient receptor potential vanilloid 1 (TRPV1). These compounds include cervinomycins (48-55), citreamycins (56-57), sattahipmycin (59), and chrexanthomycins (60-63). This review gives succinct information on the γ-pyrone containing natural products isolated from Streptomyces sp. focusing on their structure and bioactivities. KEY POINTS: • The Streptomyces sp. is the producer of various bioactive natural products including the one with γ-pyrone ring. • These γ-pyrone compounds are structurally different and possess different bioactivities. • The Streptomyces has the potential to produce such compounds and the reservoir of these compounds is expected to increase in the future.
Keywords: Streptomyces; Anthra-γ-pyrone; Nitrophenyl-γ-pyrone; Pluramycin; γ-Pyrone.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Shellmycin A-D, Novel Bioactive Tetrahydroanthra-γ-Pyrone Antibiotics from Marine Streptomyces sp. Shell-016.Mar Drugs. 2020 Jan 16;18(1):58. doi: 10.3390/md18010058. Mar Drugs. 2020. PMID: 31963176 Free PMC article.
-
Rubiflavin G, photorubiflavin G, and photorubiflavin E: Novel pluramycin derivatives from Streptomyces sp. W2061 and their anticancer activity against breast cancer cells.J Antibiot (Tokyo). 2023 Oct;76(10):585-591. doi: 10.1038/s41429-023-00643-w. Epub 2023 Jul 6. J Antibiot (Tokyo). 2023. PMID: 37414938
-
α-Pyrone Polyketides from Streptomyces ambofaciens BI0048, an Endophytic Actinobacterial Strain Isolated from the Red Alga Laurencia glandulifera.Mar Drugs. 2017 Dec 14;15(12):389. doi: 10.3390/md15120389. Mar Drugs. 2017. PMID: 29240664 Free PMC article.
-
2-Pyrone natural products and mimetics: isolation, characterisation and biological activity.Nat Prod Rep. 2005 Jun;22(3):369-85. doi: 10.1039/b416651p. Epub 2005 May 5. Nat Prod Rep. 2005. PMID: 16010346 Review.
-
Endophytic Streptomyces: an underexplored source with potential for novel natural drug discovery and development.Arch Microbiol. 2024 Oct 22;206(11):442. doi: 10.1007/s00203-024-04169-z. Arch Microbiol. 2024. PMID: 39436470 Review.
References
-
- Bhat ZS, Rather MA, Maqbool M, Lah HU, Yousuf SK, Ahmad Z (2017) α-pyrones: small molecules with versatile structural diversity reflected in multiple pharmacological activities-an update. Biomed Pharmacother 91:265–277. 10.1016/j.biopha.2017.04.012 - PubMed
-
- Busch B, Hertweck C (2009) Evolution of metabolic diversity in polyketide-derived pyrones: using the non-colinear aureothin assembly line as a model system. Phytochemistry 70:1833–1840. 10.1016/j.phytochem.2009.05.022 - PubMed
-
- Chang Y, Xing L, Sun C, Liang S, Liu T, Zhang X, Zhu T, Pfeifer BA, Che Q, Zhang G, Li D (2020) Monacycliones G−K and ent-gephyromycin A, angucycline derivatives from the marine-derived Streptomyces sp. HDN15129. J Nat Prod 83:2749–2755. 10.1021/acs.jnatprod.0c00684 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

