Plant developmental oddities
- PMID: 39316298
- PMCID: PMC11422487
- DOI: 10.1007/s00425-024-04534-8
Plant developmental oddities
Abstract
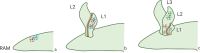
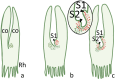
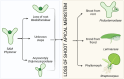
Plants lacking shoot apical meristem develop with unique body shapes, suggesting rewiring of developmental genes. This loss of the meristem is likely influenced by a combination of environmental factors and evolutionary pressures. This study explores the development of plant bodies in three families (Podostemaceae, Lemnaceae, and Gesneriaceae) where the shoot apical meristem (SAM), a key structure for growth, is absent or altered. The review highlights alternative developmental strategies these plants employ. Also, we considered alternative reproduction in those species, namely through structures like turions, fronds, or modified leaves, bypassing the need for a SAM. Further, we report on studies based on the expression patterns of genes known to be involved in SAM formation and function. Interestingly, these genes are still present but expressed in atypical locations, suggesting a rewiring of developmental networks. Our view on the current literature and knowledge indicates that the loss or reduction of the SAM is driven by a combination of environmental pressures and evolutionary constraints, leading to these unique morphologies. Further research, also building on Next-Generation Sequencing, will be instrumental to explore the genetic basis for these adaptations and how environmental factors influence them.
Keywords: Bauplan; Evolutionary adaptation; Phytomer; Plant development; SAM.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that there is no competing interest.
Figures

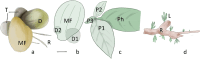


References
-
- Abramson BW, Novotny M, Hartwick NT, Colt K, Aevermann BD, Scheuermann RH, Michael TP (2021) Single nuclei transcriptome of the Lesser Duckweed Lemna minuta reveals cell trajectories for an entire plant. bioRxiv. 10.1101/2021.06.03.446947
-
- Agarwal Y, Shukla B, Manivannan A, Soundararajan P (2022) Paradigm and framework of WUS-CLV feedback loop in stem cell niche for SAM maintenance and cell identity transition. Agronomy 12(12):3132. 10.3390/agronomy12123132
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

