Kidney Injury Evoked by Fine Particulate Matter: Risk Factor, Causation, Mechanism and Intervention Study
- PMID: 39316383
- PMCID: PMC11578332
- DOI: 10.1002/advs.202403222
Kidney Injury Evoked by Fine Particulate Matter: Risk Factor, Causation, Mechanism and Intervention Study
Abstract
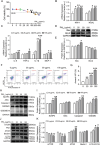
Fine particulate matter (PM2.5) is suggested to pose a severe risk to the kidneys by inducing functional degradation and chronic kidney diseases (CKD). This study aims to explore the nephrotoxicity of PM2.5 exposure and the underlying mechanism. Herein, based on the UK Biobank, it is found that per interquartile range (IQR) increase in PM2.5 is associated with a 6% (95% CI: 1%-11%), 7% (95% CI: 3%-11%), 9% (95% CI: 4%-13%), 11% (95% CI: 9%-13%), and 10% (95% CI: 8%-12%) increase in the risk of nephritis, hydronephrosis, kidney stone, acute renal failure, and CKD, respectively. In experimental study, noticeable kidney injury, which is the initiation of kidney diseases, is observed with PM2.5 exposure in C57BL/6N mice (n = 8), accompanied with oxidative stress, autophagy and pyroptosis. In vitro, HK-2 cells with PM2.5-stimulation exhibit tubulopathy, increased reactive oxygen species (ROS) generation and activated pyroptosis and autophagy. All changes are abolished by ROS scavenger of N-acetyl-L-cysteine (NAC) both in vivo and in vitro. In conclusion, the study provides evidence showing that PM2.5 exposure is associated with 5 kinds of kidney diseases by directly inducing nephrotoxicity, in which ROS may be the potential target by triggering autophagy and pyroptosis.
Keywords: autophagy; fine particulate matter; kidney injury; oxidative stress; pyroptosis.
© 2024 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
-
- a) Liu F., Chen G., Huo W., Wang C., Liu S., Li N., Mao S., Hou Y., Lu Y., Xiang H., Environ. Pollut. 2019, 252, 1235; - PubMed
- b) Yitshak‐Sade M., Nethery R., Schwartz J. D., Mealli F., Dominici F., Di Q., Abu Awad Y., Ifergane G., Zanobetti A., Sci. Total Environ. 2021, 755, 142524. - PMC - PubMed
-
- a) Lin S. Y., Ju S. W., Lin C. L., Hsu W. H., Lin C. C., Ting I. W., Kao C. H., Environ. Pollut. 2020, 261, 114154; - PubMed
- b) Bowe B., Artimovich E., Xie Y., Yan Y., Cai M., Al‐Aly Z., BMJ Glob Health 2020, 5, e002063; - PMC - PubMed
- c) Li G., Huang J., Wang J., Zhao M., Liu Y., Guo X., Wu S., Zhang L., J. Am. Soc. Nephrol. 2021, 32, 448; - PMC - PubMed
- d) Wu Y. H., Wu C. D., Chung M. C., Chen C. H., Wu L. Y., Chung C. J., Hsu H. T., Front. Public Health 2022, 10, 858655. - PMC - PubMed
-
- Romagnani P., Remuzzi G., Glassock R., Levin A., Jager K. J., Tonelli M., Massy Z., Wanner C., Anders H. J., Nat. Rev. Dis. Primers 2017, 3, 17088. - PubMed
-
- Jourde‐Chiche N., Fakhouris F., Dou L., Bellien J., Burtey S., Frimat M., Jarrot P. A., Kaplanski G., Le Quintrec M., Pernin V., Rigothier C., Sallée M., Fremeaux‐Bacchi V., Guerrot D., Roumenina L. T., Nat. Rev. Nephrol. 2019, 15, 87. - PubMed
-
- Yao L., Liang X., Qiao Y., Chen B., Wang P., Liu Z., Metabolism. 2022, 131, 155195. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
