Barriers and facilitators to next-generation sequencing use in United States oncology settings: a systematic review
- PMID: 39316553
- PMCID: PMC11572137
- DOI: 10.1080/14796694.2024.2390821
Barriers and facilitators to next-generation sequencing use in United States oncology settings: a systematic review
Abstract
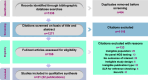
Aim: Next-generation sequencing (NGS) of solid tumors can inform treatment decisions; however, uptake remains low. This objective of this systematic review was to identify barriers to and facilitators of NGS in US oncology settings.Materials & methods: Embase and MEDLINE were searched in March 2023 for articles published from 2012 to 2023 on barriers and facilitators of NGS adoption for solid tumors. Surveys, interviews and observational studies were eligible. Studies on genetic testing for hereditary cancers and non-US studies were excluded. The Motheral scale, Joanna Briggs Institute critical appraisal checklist and McGill Mixed Methods Appraisal Tool were used to assess study quality. Data were synthesized narratively.Results: Twenty-one studies were included. Study participants were clinicians, payers and administrators. Key barriers included complex reimbursement processes and uncertainties around clinical utility. Including recommendations for NGS in clinical practice guidelines was a key facilitator, although insurance policies were often more restrictive than guideline recommendations.Conclusion: Uptake of NGS is increasing but barriers remain. Changes to the current reimbursement frameworks are needed to increase access to NGS. The impact of implementing the 2018 National Coverage Determination, which allows access to NGS for all Medicare beneficiaries with advanced cancer, is not yet evident in the published literature.
Keywords: clinical oncology; genetic testing; high-throughput sequencing; massive parallel sequencing; next-generation sequencing; precision medicine; qualitative evidence synthesis; systematic review.
Plain language summary
[Box: see text].
Conflict of interest statement
G Ko, S Appukkuttan, B Hocum and S Babajanyan are employed by Bayer while SMA is a former employee of Bayer. A Ferreira-Gonzalez is a consultant for Bayer. N Fusco, F Stewart and K Kistler are employed by Cencora. The authors have no other competing interests or relevant affiliations with any organization or entity with the subject matter or materials discussed in the manuscript apart from those disclosed.
Figures

References
-
- Li MM, Datto M, Duncavage EJ, et al. . Standards and Guidelines for the Interpretation and Reporting of Sequence Variants in Cancer: A Joint Consensus Recommendation of the Association for Molecular Pathology, American Society of Clinical Oncology, and College of American Pathologists. J Mol Diagn. 2017;19(1):4–23. doi:10.1016/j.jmoldx.2016.10.002 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
