Drosophila larvae form appetitive and aversive associative memory in response to thermal conditioning
- PMID: 39316589
- PMCID: PMC11421805
- DOI: 10.1371/journal.pone.0303955
Drosophila larvae form appetitive and aversive associative memory in response to thermal conditioning
Abstract
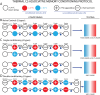
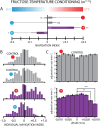
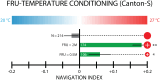
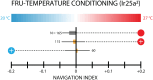
Organisms have evolved the ability to detect, process, and respond to many different surrounding stimuli in order to successfully navigate their environments. Sensory experiences can also be stored and referenced in the form of memory. The Drosophila larva is a simple model organism that can store associative memories during classical conditioning, and is well-suited for studying learning and memory at a fundamental level. Much progress has been made in understanding larval learning behavior and the associated neural circuitry for olfactory conditioning, but other sensory systems are relatively unexplored. Here, we investigate memory formation in larvae treated with a temperature-based associative conditioning protocol, pairing normally neutral temperatures with appetitive (fructose, FRU) or aversive (salt, NaCl) stimuli. We test associative memory using thermal gradient geometries, and quantify navigation strength towards or away from conditioned temperatures. We find that larvae demonstrate short-term associative learning. They navigate towards warmer or cooler temperatures paired with FRU, and away from warmer or cooler temperatures paired with NaCl. These results, especially when combined with future investigations of thermal memory circuitry in larvae, should provide broader insight into how sensory stimuli are encoded and retrieved in insects and more complex systems.
Copyright: © 2024 Polizos et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

