Wnt target gene activation requires β-catenin separation into biomolecular condensates
- PMID: 39316611
- PMCID: PMC11460698
- DOI: 10.1371/journal.pbio.3002368
Wnt target gene activation requires β-catenin separation into biomolecular condensates
Abstract
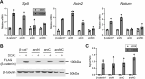
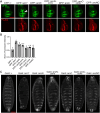
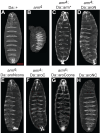
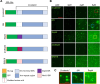
The Wnt/β-catenin signaling pathway plays numerous essential roles in animal development and tissue/stem cell maintenance. The activation of genes regulated by Wnt/β-catenin signaling requires the nuclear accumulation of β-catenin, a transcriptional co-activator. β-catenin is recruited to many Wnt-regulated enhancers through direct binding to T-cell factor/lymphoid enhancer factor (TCF/LEF) family transcription factors. β-catenin has previously been reported to form phase-separated biomolecular condensates (BMCs), which was implicated as a component of β-catenin's mechanism of action. This function required aromatic amino acid residues in the intrinsically disordered regions (IDRs) at the N- and C-termini of the protein. In this report, we further explore a role for β-catenin BMCs in Wnt target gene regulation. We find that β-catenin BMCs are miscible with LEF1 BMCs in vitro and in cultured cells. We characterized a panel of β-catenin mutants with different combinations of aromatic residue mutations in human cell culture and Drosophila melanogaster. Our data support a model in which aromatic residues across both IDRs contribute to BMC formation and signaling activity. Although different Wnt targets have different sensitivities to loss of β-catenin's aromatic residues, the activation of every target examined was compromised by aromatic substitution. These mutants are not defective in nuclear import or co-immunoprecipitation with several β-catenin binding partners. In addition, residues in the N-terminal IDR with no previously known role in signaling are clearly required for the activation of various Wnt readouts. Consistent with this, deletion of the N-terminal IDR results in a loss of signaling activity, which can be rescued by the addition of heterologous IDRs enriched in aromatic residues. Overall, our work supports a model in which the ability of β-catenin to form biomolecular condensates in the nucleus is tightly linked to its function as a transcriptional co-regulator.
Copyright: © 2024 Stewart et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

