Sustained benefit of zanubrutinib vs ibrutinib in patients with R/R CLL/SLL: final comparative analysis of ALPINE
- PMID: 39316666
- PMCID: PMC11738031
- DOI: 10.1182/blood.2024024667
Sustained benefit of zanubrutinib vs ibrutinib in patients with R/R CLL/SLL: final comparative analysis of ALPINE
Abstract
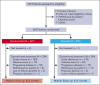
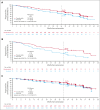
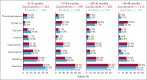
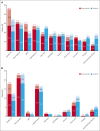
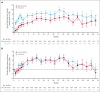
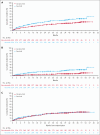
The ALPINE trial established the superiority of zanubrutinib over ibrutinib in patients with relapsed/refractory chronic lymphocytic leukemia and small lymphocytic lymphoma; here, we present data from the final comparative analysis with extended follow-up. Overall, 652 patients received zanubrutinib (n = 327) or ibrutinib (n = 325). At an overall median follow-up of 42.5 months, progression-free survival benefit with zanubrutinib vs ibrutinib was sustained (hazard ratio [HR], 0.68; 95% confidence interval [CI], 0.54-0.84), including in patients with del(17p)/TP53 mutation (HR, 0.51; 95% CI, 0.33-0.78) and across multiple sensitivity analyses. Overall response rate remained higher with zanubrutinib compared with ibrutinib (85.6% vs 75.4%); responses deepened over time with complete response/complete response with incomplete bone marrow recovery rates of 11.6% (zanubrutinib) and 7.7% (ibrutinib). Although median overall survival has not been reached in either treatment group, fewer zanubrutinib patients have died than ibrutinib patients (HR, 0.77 [95% CI, 0.55-1.06]). With median exposure time of 41.2 and 37.8 months in zanubrutinib and ibrutinib arms, respectively, the most common nonhematologic adverse events included COVID-19-related infection (46.0% vs 33.3%), diarrhea (18.8% vs 25.6%), upper respiratory tract infection (29.3% vs 19.8%), and hypertension (27.2% vs 25.3%). Cardiac events were lower with zanubrutinib (25.9% vs 35.5%) despite similar rates of hypertension. Incidence of atrial fibrillation/flutter was lower with zanubrutinib vs ibrutinib (7.1% vs 17.0%); no cardiac deaths were reported with zanubrutinib vs 6 cardiac deaths with ibrutinib. This analysis, at 42.5 months median follow-up, demonstrates that zanubrutinib remains more efficacious than ibrutinib with an improved overall safety/tolerability profile. This trial was registered at www.ClinicalTrials.gov as #NCT03734016.
© 2024 American Society of Hematology. Published by Elsevier Inc. All rights are reserved, including those for text and data mining, AI training, and similar technologies.
Conflict of interest statement
Conflicts-of-interest disclosure: J.R.B. reports consultancy with AbbVie, Acerta/AstraZeneca, Alloplex Biotherapeutics, BeiGene, Galapagos NV, Genentech/Roche, Grifols Worldwide Operations, InnoCare Pharma Inc, iOnctura, Kite, Loxo/Lilly, Merck, Numab Therapeutics, Pfizer, and Pharmacyclics; and research funding from BeiGene, Gilead, iOnctura, Loxo/Lilly, MEI Pharma, TG Therapeutics. B.E. reports research funding from Janssen, Gilead, Roche, AbbVie, BeiGene, and AstraZeneca; received honoraria from Janssen, BeiGene, Roche, AbbVie, Novartis, Celgene, and AstraZeneca; serves on speakers bureau for Roche, AbbVie, Merck Sharp & Dohme, BeiGene, and AstraZeneca; and received travel, accommodations, and expenses funding from BeiGene. N.L. reports consultancy with AbbVie, AstraZeneca, BeiGene, Lilly/Loxo, Genentech, Janssen, and Pharmacyclics; and research funding from AbbVie, AstraZeneca, BeiGene, Lilly/Loxo, Genentech, Octapharma, Oncternal, MingSight, and TG Therapeutics. S.M.O. reports consultancy with AbbVie, AstraZeneca, BeiGene, Lilly, Janssen, Johnson & Johnson, Pfizer, and Pharmacyclics; research funding from BeiGene, Lilly, Pfizer, Pharmacyclics, Regeneron; and membership with the CLL Society (unpaid). C.S.T. reports research funding from Janssen, AbbVie, and BeiGene; received honoraria from Janssen, AbbVie, BeiGene, Loxo, and AstraZeneca. L.Q. reports consultancy with Janssen, AstraZeneca, Takeda, Roche, AbbVie, and BeiGene. W.J. reports consultancy with Janssen, AstraZeneca, MEI Pharma, Lilly, Takeda, Roche, AbbVie, and BeiGene; reports research funding: from AbbVie, Bayer, BeiGene, Celgene, Janssen, Roche, Takeda, TG Therapeutics, AstraZeneca, MEI Pharma, and Lilly. M. Simkovic received consulting fees from AbbVie, AstraZeneca, and Janssen-Cilag; reports individual stocks in AbbVie, AstraZeneca, Johnson & Johnson, BeiGene, Gilead, Baxter, Novartis, Abbot, and Sanofi; reports honoraria from AbbVie, Janssen-Cilag, and AstraZeneca; reports membership on an entity’s board of directors or advisory committees of AbbVie, Janssen-Cilag, and AstraZeneca; and received travel, accommodations, and expenses funding from AbbVie, Janssen-Cilag, and AstraZeneca. J.M. received research funding from BeiGene. A.F. received honoraria from AbbVie, AstraZeneca, and Genentech; and reports research funding from AbbVie, AstraZeneca, BeiGene, and Genmab. R.W. reports honoraria from Janssen, AbbVie, and BeiGene; received research funding from BioOra; and is a current equity holder in the publicly traded company Fisher & Paykel Healthcare. T.R. reports honoraria from AbbVie, Janssen, BeiGene, AstraZeneca, Octopharma, Regeneron, and GlaxoSmithKline; reports consultancy with Janssen, BeiGene, and AstraZeneca; and received research funding from Janssen, BeiGene, AstraZeneca, OctoPharma, Regeneron, and GlaxoSmithKline. H.A.Y. serves on the speakers bureau for AbbVie, Amgen, AstraZeneca, BeiGene, GlaxoSmithKline, Jenssen, Karyopharm, and Takeda. M.W., T.S., L.W., J.L., and K.W. are employed by BeiGene; and are equity holders in BeiGene. A.C. was an employee of BeiGene at the time of the study, which included equity holder, and funds for travel, accommodations, and expenses. M. Shadman reports consultancy with, and roles on advisory boards, steering committees, or data safety monitoring committees of, AbbVie, Genentech, AstraZeneca, Genmab, Janssen, BeiGene, Bristol Myers Squibb, Morphosys/Incyte, Kite Pharma, Eli Lilly, Mustang Bio, Fate therapeutics, Nurix, and Merck; reports institutional research funding from Mustang Bio, Genentech, AbbVie, BeiGene, AstraZeneca, Genmab, MorphoSys/Incyte, and Vincerx; reports stock options in Koi Biotherapeutics; and reports employment by Bristol Myers Squibb (spouse). The remaining authors declare no competing financial interests.
Figures


References
-
- Imbruvica. Package insert. Pharmacyclics LLC; 2022.
-
- Shadman M, Flinn IW, Levy MY, et al. Zanubrutinib in patients with previously treated B-cell malignancies intolerant of previous Bruton tyrosine kinase inhibitors in the USA: a phase 2, open-label, single-arm study. Lancet Haematol. 2023;10(1):e35–e45. - PubMed
-
- Ou YC, Tang Z, Novotny W, et al. Rationale for once-daily or twice-daily dosing of zanubrutinib in patients with mantle cell lymphoma. Leuk Lymphoma. 2021;62(11):2612–2624. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

