Effects of glial-derived neurotrophic factor on remodeling and mitochondrial function in human airway smooth muscle cells
- PMID: 39316680
- PMCID: PMC11563586
- DOI: 10.1152/ajplung.00101.2024
Effects of glial-derived neurotrophic factor on remodeling and mitochondrial function in human airway smooth muscle cells
Abstract
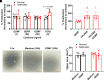
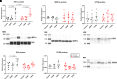
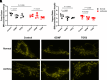
Airway smooth muscle (ASM) cells play important roles in airway remodeling of asthma. Our previous studies show that in vivo administration of glial-derived neurotrophic factor (GDNF) in mice induces thickening and collagen deposition in bronchial airways, whereas chelation of GDNF by GFRα1-Fc attenuates airway remodeling in the context of allergen exposure. To determine whether GDNF has direct effects on ASM, in this study, we examined GDNF in ASM cells from normal versus asthmatic humans. We found that GDNF treatment of human ASM cells had only minor effects on cell proliferation and migration, intracellular expression or extracellular deposition of collagen I (COL1), collagen III (COL3), and fibronectin. Endoplasmic reticulum (ER) stress response and mitochondrial function have been implicated in asthma. We investigated whether GDNF regulates these aspects in human ASM. We found that GDNF treatment did not affect ER stress protein expression in normal or asthmatic cells. However, GDNF treatment impaired mitochondrial morphology in ASM but without significant effects on mitochondrial respiration. Thus, it is likely that in vivo effects of GDNF on airway remodeling per se involve cell types other than those on ASM, and thus ASM may serve more as a source of GDNF rather than a target.NEW & NOTEWORTHY Our previous study suggests that glial-derived neurotrophic factor (GDNF) is involved in allergen-induced airway hyperreactivity and remodeling in vivo. Here, we show that GDNF has no direct effects in remodeling of human airway smooth muscle (ASM) but GDNF dysregulates mitochondrial morphology in human ASM in the context of asthma.
Keywords: airway smooth muscle; asthma; mitochondria; neurotrophin; remodeling.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

