Dual ablation of the RyR2-Ser2808 and RyR2-Ser2814 sites increases propensity for pro-arrhythmic spontaneous Ca2+ releases
- PMID: 39316734
- PMCID: PMC11493507
- DOI: 10.1113/JP286453
Dual ablation of the RyR2-Ser2808 and RyR2-Ser2814 sites increases propensity for pro-arrhythmic spontaneous Ca2+ releases
Abstract
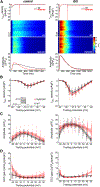
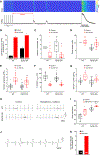
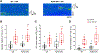
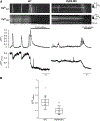
During exercise or stress, the sympathetic system stimulates cardiac contractility via β-adrenergic receptor (β-AR) activation, resulting in phosphorylation of the cardiac ryanodine receptor (RyR2). Three RyR2 phosphorylation sites have taken prominence in excitation-contraction coupling: S2808 and S2030 are described as protein kinase A specific and S2814 as a Ca2+/calmodulin kinase type-2-specific site. To examine the contribution of these phosphosites to Ca2+ signalling, we generated double knock-in (DKI) mice in which Ser2808 and Ser2814 phosphorylation sites have both been replaced by alanine (RyR2-S2808A/S2814A). These mice did not exhibit an overt phenotype. Heart morphology and haemodynamic parameters were not altered. However, they had a higher susceptibility to arrhythmias. We performed confocal Ca2+ imaging and electrophysiology experiments. Isoprenaline was used to stimulate β-ARs. Measurements of Ca2+ waves and latencies in myocytes revealed an increased propensity for spontaneous Ca2+ releases in DKI myocytes, both in control conditions and during β-AR stimulation. In DKI cells, waves were initiated from a lower threshold concentration of Ca2+ inside the sarcoplasmic reticulum, suggesting higher Ca2+ sensitivity of the RyRs. The refractoriness of Ca2+ spark triggering depends on the Ca2+ sensitivity of the RyR2. We found that RyR2-S2808A/S2814A channels were more Ca2+ sensitive in control conditions. Isoprenaline further shortened RyR refractoriness in DKI cardiomyocytes. Together, our results suggest that ablation of both the RyR2-Ser2808 and RyR2-S2814 sites increases the propensity for pro-arrhythmic spontaneous Ca2+ releases, as previously suggested for hyperphosphorylated RyRs. Given that the DKI cells present a full response to isoprenaline, the data suggest that phosphorylation of Ser2030 might be sufficient for β-AR-mediated sensitization of RyRs. KEY POINTS: Phosphorylation of cardiac sarcoplasmic reticulum Ca2+-release channels (ryanodine receptors, RyRs) is involved in the regulation of cardiac function. Ablation of both the RyR2-Ser2808 and RyR2-Ser2814 sites increases the propensity for pro-arrhythmic spontaneous Ca2+ releases, as previously suggested for hyperphosphorylated RyRs. The intra-sarcoplasmic reticulum Ca2+ threshold for spontaneous Ca2+ wave generation is lower in RyR2-double-knock-in cells. The RyR2 from double-knock-in cells exhibits increased Ca2+ sensitivity. Phosphorylation of Ser2808 and Ser2814 might be important for basal activity of the channel. Phosphorylation of Ser2030 might be sufficient for a β-adrenergic response.
Keywords: Ca2+‐induced Ca2+ release; cardiac muscle; dephosphorylation; excitation–contraction coupling; protein phosphatase; ryanodine receptor 2.
© 2024 The Author(s). The Journal of Physiology published by John Wiley & Sons Ltd on behalf of The Physiological Society.
Conflict of interest statement
Competing interests:
The authors declare no competing interests.
Figures




References
-
- Bankhead P, Scholfield CN, Curtis TM & McGeown JG (2011). Detecting Ca2+ sparks on stationary and varying baselines. Am J Physiol Cell Physiol 301, C717–28. - PubMed
-
- Benkusky NA, Weber CS, Scherman JA, Farrell EF, Hacker TA, John MC, Powers PA & Valdivia HH (2007). Intact beta-adrenergic response and unmodified progression toward heart failure in mice with genetic ablation of a major protein kinase A phosphorylation site in the cardiac ryanodine receptor. Circ Res 101, 819–829. - PubMed
-
- Bers DM (2002). Cardiac excitation-contraction coupling. Nature 415, 198–205. - PubMed
-
- Bers DM & Berlin JR (1995). Kinetics of [Ca]i decline in cardiac myocytes depend on peak [Ca]i. Am J Physiol-Cell Physiol 268, C271–C277. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

