Regulation of macrophage fibrinolysis during venous thrombus resolution
- PMID: 39317013
- PMCID: PMC11486561
- DOI: 10.1016/j.thromres.2024.109149
Regulation of macrophage fibrinolysis during venous thrombus resolution
Abstract
Background: Venous thromboembolism (VTE), which includes pulmonary embolism (PE) and deep vein thrombosis (DVT), is a serious cardiovascular disease with significant mortality and morbidity. Clinically, patients with faster resolution of a venous thrombi have improved prognosis. Urokinase-plasminogen activator (uPA), produced by macrophages, is a key mediator of fibrinolysis required for resolving venous thrombi and restoring vascular integrity. The major macrophage protein, plasminogen activator inhibitor type-2 (PAI-2), was originally identified as an inhibitor of uPA and is implicated in the modulation of pathways affecting fibrinolytic uPA activity, however its direct role in blocking uPA-mediated clot lysis is not known.
Objective: To determine the contribution of macrophage PAI-2 in inhibiting uPA-mediated fibrinolysis during resolution of DVT.
Methods: Using a murine model of venous thrombosis and resolution, we determined histological changes and molecular features of fibrin degradation in venous thrombi from WT mice and mice genetically deficient in PAI-2 and PAI-1, and determined the fibrinolytic activities of macrophages from these genotypes ex vivo.
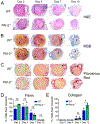
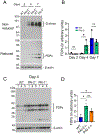
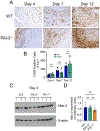
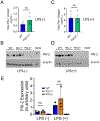
Results: Acceleration of venous thrombus resolution by PAI-2-/- mice increases fibrin degradation in venous thrombi showing a pattern similar to genetic deficiency of PAI-1, the major attenuator of fibrinolysis. PAI-2 deficiency was not associated with increased macrophage infiltration into thrombi or changes in macrophage PAI-1 expression. uPA-initiated fibrinolysis by macrophages in vitro could be accelerated by PAI-1 deficiency, but not PAI-2 deficiency.
Conclusion: PAI-2 has an alternate anti-fibrinolytic activity that is macrophage uPA independent, where PAI-1 is the dominant uPA inhibitor during DVT resolution.
Keywords: Fibrin; Fibrinolysis; Macrophage; PAI-1; PAI-2; Plasminogen activator inhibitor; Venous thrombosis.
Published by Elsevier Ltd.
Conflict of interest statement
Declaration of competing interest There are no competing interests to disclose.
Figures


References
-
- Meissner MH, Manzo RA, Bergelin RO, Markel A, Strandness DE Jr. Deep venous insufficiency: the relationship between lysis and subsequent reflux. J Vasc Surg. 1993;18(4):596–605; discussion 6-8. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

