MicroRNA-376a-3p sensitizes CPT-11-resistant colorectal cancer by enhancing apoptosis and reversing the epithelial-to-mesenchymal transition (EMT) through the IGF1R/PI3K/AKT pathway
- PMID: 39317064
- PMCID: PMC11456798
- DOI: 10.1016/j.tranon.2024.102125
MicroRNA-376a-3p sensitizes CPT-11-resistant colorectal cancer by enhancing apoptosis and reversing the epithelial-to-mesenchymal transition (EMT) through the IGF1R/PI3K/AKT pathway
Abstract
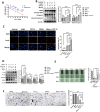
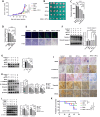
Colorectal cancer (CRC) remains the third most prevalent type of cancer worldwide contributing to an estimated 10 % of all cancer cases. CPT-11 is one of the first-line drugs for CRC treatment. Unfortunately, the development of drug resistance significantly exacerbates the adverse impact of CRC. Consequent tumor recurrences and metastasis, years after treatment are the frequently reported incidences. MicroRNAs (miRNA) are short non-coding RNA with the functionality of gene suppression. The insulin-like growth factor type 1 receptor (IGF1R) is a tyrosine kinase receptor frequently upregulated in cancers and is associated with cell survival and drug resistance. MiRNAs are frequently reported to be dysregulated in cancers including CRC. Evidence suggests that dysregulated miRNAs have direct consequences on the biological processes of their target genes. We previously demonstrated that miRNA-376a-3p is upregulated in CPT-11responsive, CRC cells upon treatment with CPT-11. We therefore aimed to investigate the involvement of miRNA-376a-3p in CPT-11 resistance and its probable association with IGF1R-mediated cancer cell survival. Our experimental approach used knockdown and overexpression experiments supplemented with western blot, RT-qPCR, flow cytometry, MTT, and migration assays to achieve our aim. Our data reveals the mechanism through which IGF1R and miRNA-376a-3p perpetrate and attenuate CPT-11 resistance respectively. MiRNA-376a-3p overexpression negatively regulated the IGF1R-induced cell survival, PI3K/AKT pathway, and reversed the epithelial-mesenchymal transition, hence sensitizing resistant cells to CPT-11. Our findings suggests that the miRNA-376a-3p/IGF1R axis holds promise as a potential target to sensitize CRC to CPT-11 in cases of drug resistance.
Keywords: CPT-11; CPT-11 resistance; Colorectal cancer; IGF1R; miRNA-376a-3p.
Copyright © 2024. Published by Elsevier Inc.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures






References
-
- Sung H., et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 Countries. CA Cancer J. Clin. 2021;71(3):209–249. - PubMed
-
- Biller L.H., Schrag D. Diagnosis and treatment of metastatic colorectal cancer: a review. JAMA. 2021;325(7):669–685. - PubMed
LinkOut - more resources
Full Text Sources

