Substrate-Multiplexed Assessment of Aromatic Prenyltransferase Activity
- PMID: 39317170
- PMCID: PMC11727010
- DOI: 10.1002/cbic.202400680
Substrate-Multiplexed Assessment of Aromatic Prenyltransferase Activity
Abstract
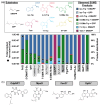
An increasingly effective strategy to identify synthetically useful enzymes is to sample the diversity already present in Nature. Here, we construct and assay a panel of phylogenetically diverse aromatic prenyltransferases (PTs). These enzymes catalyze a variety of C-C bond forming reactions in natural product biosynthesis and are emerging as tools for synthetic chemistry and biology. Homolog screening was further empowered through substrate-multiplexed screening, which provides direct information on enzyme specificity. We perform a head-to-head assessment of the model members of the PT family and further identify homologs with divergent sequences that rival these superb enzymes. This effort revealed the first bacterial O-Tyr PT and, together, provide valuable benchmarking for future synthetic applications of PTs.
Keywords: Biocatalysis; Enzyme Promiscuity; Multiplexed Screening; Sequence-similarity Network; Substrate Scope.
© 2024 The Author(s). ChemBioChem published by Wiley-VCH GmbH.
Conflict of interest statement
ARB is an inventor on a patent for the synthetic applications of engineered tryptophan synthases.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

