Signal Detection Theoretic Estimates of the Murine Absolute Visual Threshold Are Independent of Decision Bias
- PMID: 39317466
- PMCID: PMC11470389
- DOI: 10.1523/ENEURO.0222-24.2024
Signal Detection Theoretic Estimates of the Murine Absolute Visual Threshold Are Independent of Decision Bias
Abstract
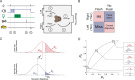
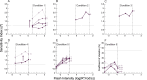
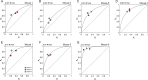
Decision bias influences estimates of the absolute visual threshold. However, most psychophysical estimates of the murine absolute visual threshold have not taken bias into account. Here we developed a one-alternative forced choice (1AFC) assay to assess the decision bias of mice at the absolute visual threshold via the theory of signal detection and compared our approach with the more conventional high-threshold theoretic approach. In the 1AFC assay, mice of both sexes were trained to signal whether they detected a flash stimulus. We directly measured both hit and false alarm rates, which were used to estimate d' Using the theory of signal detection, we obtained absolute thresholds by interpolating the intensity where d' = 1 from d'-psychometric functions. This gave bias-independent estimates of the absolute visual threshold which ranged over sixfold, averaging ∼1 R* in 1,000 rods (n = 7 mice). To obtain high-threshold theoretic estimates of the absolute visual threshold from the same mice, we estimated threshold intensities from the frequency of seeing curves, corrected for guessing. This gave us thresholds that were strongly correlated with decision bias, ranging over 13-fold and averaged ∼1 R* in 2,500 rods. We conclude that the theory of signal detection uses false alarms to overcome decision bias and narrow the range of threshold estimates in mice, providing a powerful tool for understanding detection behavior near absolute visual threshold.
Keywords: absolute visual thresholds; mouse vision; operant behavior; response bias; theory of signal detection; visual noise.
Copyright © 2024 LaMagna et al.
Conflict of interest statement
The authors declare no competing financial interests.
Figures





Similar articles
-
Contribution of Sensory Encoding to Measured Bias.J Neurosci. 2019 Jun 26;39(26):5115-5127. doi: 10.1523/JNEUROSCI.0076-19.2019. Epub 2019 Apr 23. J Neurosci. 2019. PMID: 31015339 Free PMC article.
-
Suprathreshold stochastic resonance in visual signal detection.Behav Brain Res. 2008 Nov 3;193(1):152-5. doi: 10.1016/j.bbr.2008.05.003. Epub 2008 May 8. Behav Brain Res. 2008. PMID: 18565603 Clinical Trial.
-
Visual Temporal Contrast Sensitivity in the Behaving Mouse Shares Fundamental Properties with Human Psychophysics.eNeuro. 2018 Aug 29;5(4):ENEURO.0181-18.2018. doi: 10.1523/ENEURO.0181-18.2018. eCollection 2018 Jul-Aug. eNeuro. 2018. PMID: 30225342 Free PMC article.
-
Visual Decision-Making in an Uncertain and Dynamic World.Annu Rev Vis Sci. 2017 Sep 15;3:227-250. doi: 10.1146/annurev-vision-111815-114511. Epub 2017 Jul 17. Annu Rev Vis Sci. 2017. PMID: 28715956 Review.
-
Expectation in perceptual decision making: neural and computational mechanisms.Nat Rev Neurosci. 2014 Nov;15(11):745-56. doi: 10.1038/nrn3838. Epub 2014 Oct 15. Nat Rev Neurosci. 2014. PMID: 25315388 Review.
References
-
- Barlow HB (1977) Retinal and central factors in human vision limited by noise. In: Photoreception in vertebrates (Barlow HB, Fatts P, eds), pp 337–358. London: Academic Press.
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
