ATH434, a promising iron-targeting compound for treating iron regulation disorders
- PMID: 39317669
- PMCID: PMC12151019
- DOI: 10.1093/mtomcs/mfae044
ATH434, a promising iron-targeting compound for treating iron regulation disorders
Abstract
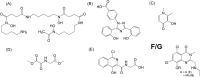
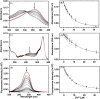
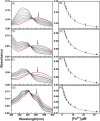
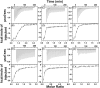
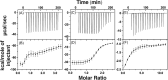
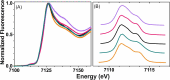
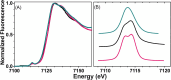
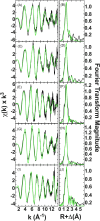
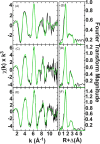
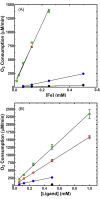
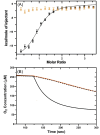
Cytotoxic accumulation of loosely bound mitochondrial Fe2+ is a hallmark of Friedreich's Ataxia (FA), a rare and fatal neuromuscular disorder with limited therapeutic options. There are no clinically approved medications targeting excess Fe2+ associated with FA or the neurological disorders Parkinson's disease and Multiple System Atrophy. Traditional iron-chelating drugs clinically approved for systemic iron overload that target ferritin-stored Fe3+ for urinary excretion demonstrated limited efficacy in FA and exacerbated ataxia. Poor treatment outcomes reflect inadequate binding to excess toxic Fe2+ or exceptionally high affinities (i.e. ≤10-31) for non-pathologic Fe3+ that disrupts intrinsic iron homeostasis. To understand previous treatment failures and identify beneficial factors for Fe2+-targeted therapeutics, we compared traditional Fe3+ chelators deferiprone (DFP) and deferasirox (DFX) with additional iron-binding compounds including ATH434, DMOG, and IOX3. ATH434 and DFX had moderate Fe2+ binding affinities (Kd's of 1-4 µM), similar to endogenous iron chaperones, while the remaining had weaker divalent metal interactions. These compounds had low/moderate affinities for Fe3+(0.46-9.59 µM) relative to DFX and DFP. While all compounds coordinated iron using molecular oxygen and/or nitrogen ligands, thermodynamic analyses suggest ATH434 completes Fe2+ coordination using H2O. ATH434 significantly stabilized bound Fe2+ from ligand-induced autooxidation, reducing reactive oxygen species (ROS) production, whereas DFP and DFX promoted production. The comparable affinity of ATH434 for Fe2+ and Fe3+ position it to sequester excess Fe2+ and facilitate drug-to-protein iron metal exchange, mimicking natural endogenous iron binding proteins, at a reduced risk of autooxidation-induced ROS generation or perturbation of cellular iron stores.
Keywords: ATH434; Iron; Iron-Targeting Compound; Regulation; Treating; Unique.
© The Author(s) 2024. Published by Oxford University Press.
Figures
References
-
- Calabrese V., Lodi R., Tonon C., D'Agata V., Sapienza M., Scapagnini G., Mangiameli A., Pennisi G., Stella A. M., Butterfield D. A., Oxidative stress, mitochondrial dysfunction and cellular stress response in Friedreich's ataxia, J. Neurol. Sci., 2005, 233(1–2), 145–162. 10.1016/j.jns.2005.03.012 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

