CAR T-cells for pediatric solid tumors: where to go from here?
- PMID: 39317919
- PMCID: PMC11554711
- DOI: 10.1007/s10555-024-10214-6
CAR T-cells for pediatric solid tumors: where to go from here?
Abstract
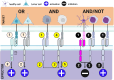
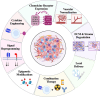
Despite the great success that chimeric antigen receptor (CAR) T-cells have had in patients with B-cell malignancies and multiple myeloma, they continue to have limited efficacy against most solid tumors. Especially in the pediatric population, pre- and post-treatment biopsies are rarely performed due to ethical reasons, and thus, our understanding is still very limited regarding the mechanisms in the tumor microenvironment by which tumor cells exclude effectors and attract immune-suppressive cells. Nevertheless, based on the principles that are known, current T-cell engineering has leveraged some of these processes and created more potent CAR T-cells. The recent discovery of new oncofetal antigens and progress made in CAR design have expanded the potential pool of candidate antigens for therapeutic development. The most promising approaches to enhance CAR T-cells are novel CAR gating strategies, creative ways of cytokine delivery to the TME without enhancing systemic toxicity, and hijacking the chemokine axis of tumors for migratory purposes. With these new modifications, the next step in the era of CAR T-cell development will be the clinical validation of these promising preclinical findings.
Keywords: Adoptive T-cell therapy; CAR; Pediatric oncology; Solid tumors.
© 2024. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
Conflict of interest statement
Figures
References
-
- Verma, M., Obergfell, K., Topp, S., Panier, V., & Wu, J. (2023). The next-generation CAR-T therapy landscape. Nature Reviews. Drug Discovery,22(10), 776–777. 10.1038/d41573-023-00140-7 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

