Inhibition of HDAC8 mitigates AKI by reducing DNA damage and promoting homologous recombination repair
- PMID: 39317961
- PMCID: PMC11422176
- DOI: 10.1111/jcmm.70114
Inhibition of HDAC8 mitigates AKI by reducing DNA damage and promoting homologous recombination repair
Abstract
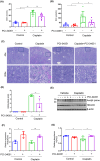
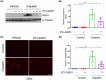
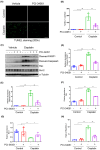
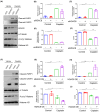
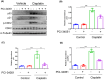
Nephrotoxicity is a major side effect of platinum-based antineoplastic drugs, and there is currently no available therapeutic intervention. Our study suggests that targeting histone deacetylase 8 could be a potential treatment for cisplatin-induced acute kidney injury (AKI). In a murine model of AKI induced by cisplatin, the administration of PCI-34051, a selective inhibitor of HDAC8, resulted in significant improvement in renal function and reduction in renal tubular damage and apoptosis. Pharmacological inhibition of HDAC8 also decreased caspase-3 and PARP1 cleavage, attenuated Bax expression and preserved Bcl-2 levels in the injured kidney. In cultured murine renal epithelial cells (mRTECs) exposed to cisplatin, treatment with PCI-34051 or transfection with HDAC8 siRNA reduced apoptotic cell numbers and diminished expression of cleaved caspase-3 and PARP1; conversely, overexpression of HDAC8 intensified these changes. Additionally, PCI-34051 reduced p53 expression levels along with those for p21, p-CDK2 and γ-H2AX while preserving MRE11 expression in the injured kidney. Similarly, pharmacological and genetic inhibition of HDAC8 reduced γ-H2AX and enhanced MRE11 expression; conversely, HDAC8 overexpression exacerbated these changes in mRTECs exposed to cisplatin. These results support that HDAC8 inhibition attenuates cisplatin-induced AKI through a mechanism associated with reducing DNA damage and promoting its repair.
Keywords: DNA damage; HDAC8; acute kidney injury; apoptosis; cisplatin; homologous recombination repair.
© 2024 The Author(s). Journal of Cellular and Molecular Medicine published by Foundation for Cellular and Molecular Medicine and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
NAM protects against cisplatin-induced acute kidney injury by suppressing the PARP1/p53 pathway.Toxicol Appl Pharmacol. 2021 May 1;418:115492. doi: 10.1016/j.taap.2021.115492. Epub 2021 Mar 17. Toxicol Appl Pharmacol. 2021. PMID: 33722665
-
Inhibition of HDAC6 protects against rhabdomyolysis-induced acute kidney injury.Am J Physiol Renal Physiol. 2017 Mar 1;312(3):F502-F515. doi: 10.1152/ajprenal.00546.2016. Epub 2017 Jan 4. Am J Physiol Renal Physiol. 2017. PMID: 28052874 Free PMC article.
-
2-Methylquinazoline derivative 23BB as a highly selective histone deacetylase 6 inhibitor alleviated cisplatin-induced acute kidney injury.Biosci Rep. 2020 Jan 31;40(1):BSR20191538. doi: 10.1042/BSR20191538. Biosci Rep. 2020. PMID: 31894849 Free PMC article.
-
Targeting DNA Damage Repair Functions of Two Histone Deacetylases, HDAC8 and SIRT6, Sensitizes Acute Myeloid Leukemia to NAMPT Inhibition.Clin Cancer Res. 2021 Apr 15;27(8):2352-2366. doi: 10.1158/1078-0432.CCR-20-3724. Epub 2021 Feb 4. Clin Cancer Res. 2021. PMID: 33542077 Free PMC article.
-
Histone deacetylases: potential therapeutic targets in cisplatin-induced acute kidney injury.Ann Med. 2024 Dec;56(1):2418958. doi: 10.1080/07853890.2024.2418958. Epub 2024 Oct 25. Ann Med. 2024. PMID: 39450927 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

