Pancreatic agenesis and altered m6A methylation in the pancreas of PDX1-mutant cynomolgus macaques
- PMID: 39318126
- PMCID: PMC11668947
- DOI: 10.24272/j.issn.2095-8137.2024.044
Pancreatic agenesis and altered m6A methylation in the pancreas of PDX1-mutant cynomolgus macaques
Abstract
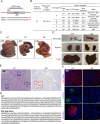
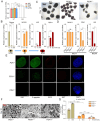
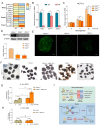
As an essential transcriptional activator, PDX1 plays a crucial role in pancreatic development and β-cell function. Mutations in the PDX1 gene may lead to type 4 maturity-onset diabetes of the young (MODY4) and neonatal diabetes mellitus. However, the precise mechanisms underlying MODY4 remain elusive due to the paucity of clinical samples and pronounced differences in pancreatic architecture and genomic composition between humans and existing animal models. In this study, three PDX1-mutant cynomolgus macaques were generated using CRISPR/Cas9 technology, all of which succumbed shortly postpartum, exhibiting pancreatic agenesis. Notably, one tri-allelic PDX1-mutant cynomolgus macaque (designated as M4) developed a pancreas, whereas the two mono-allelic PDX1-mutant cynomolgus macaques displayed no anatomical evidence of pancreatic formation. RNA sequencing of the M4 pancreas revealed substantial molecular changes in both endocrine and exocrine functions, indicating developmental delay and PDX1 haploinsufficiency. A marked change in m6A methylation was identified in the M4 pancreas, confirmed through cultured PDX1-mutant islet organoids. Notably, overexpression of the m6A modulator METTL3 restored function in heterozygous PDX1-mutant islet organoids. This study highlights a novel role of m6A methylation modification in the progression of MODY4 and provides valuable molecular insights for preclinical research.
PDX1基因对胰腺发育具有重要的调节作用,该基因突变关联少年发病的成年型糖尿病4型( Type 4 Maturity-Onset Diabetes in the Young,MODY4)或新生儿糖尿病。然而,由于临床样本的缺乏以及现有动物模型在胰腺结构和基因组与人类差异明显,导致MODY4 的发病机制尚不清晰。该研究利用 CRISPR/Cas9 技术构建了3只 PDX1 突变食蟹猴模型,然而出生不久后均很快死亡,且表现为明显的胰腺发育不全。通过基因型分析,2只猴(命名为M1和M2)的 PDX1基因为纯合突变(无明显胰腺),1只猴(命名为 M4)的 PDX1基因为三等位基因突变(表现为杂合突变),其具有胰腺组织。对M4猴胰腺组织进行RNA测序分析,结果表明其内分泌腺和外分泌腺的相关基因都发生了明显变化,且 m6A 甲基化修饰相关基因表达也发生明显变化。在体外胰岛类器官中, PDX1 杂合突变胰岛类器官也同样验证了M4猴胰腺相关基因表达差异。而当过表达 m6A 修饰蛋白 METTL3 时,可以缓解 PDX1杂合突变导致的胰岛类器官的胰岛素分泌不足。这项研究揭示了 m6A 甲基化修饰在 MODY4 胰腺发育进展过程中的新作用,并为该病的诊断和治疗提供重要的临床前研究分子基础。.
Keywords: Cynomolgus macaques; M6A methylation modification; MODY4; PDX1.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
-
- Brissova M, Dai CH, Shostak A, et al Pdx1 haploinsufficiency results in reduced MafA expression and impaired formation of beta cell secretary granules. Diabetes. 2008;57:A454.
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Research Materials
