Constitutive Androstane Receptor Regulates Germ Cell Homeostasis, Sperm Quality, and Male Fertility via Akt-Foxo1 Pathway
- PMID: 39318179
- PMCID: PMC11578384
- DOI: 10.1002/advs.202402082
Constitutive Androstane Receptor Regulates Germ Cell Homeostasis, Sperm Quality, and Male Fertility via Akt-Foxo1 Pathway
Abstract
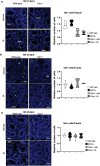
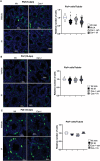
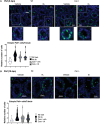
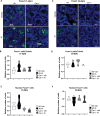
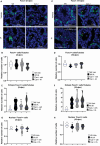
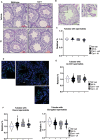
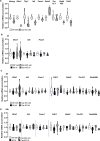
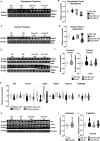
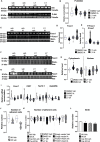
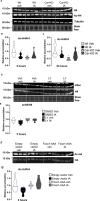
Male sexual function can be disrupted by exposure to exogenous compounds that cause testicular physiological alterations. The constitutive androstane receptor (Car) is a receptor for both endobiotics and xenobiotics involved in detoxification. However, its role in male fertility, particularly in regard to the reprotoxic effects of environmental pollutants, remains unclear. This study aims to investigate the role of the Car signaling pathway in male fertility. In vivo, in vitro, and pharmacological approaches are utilized in wild-type and Car-deficient mouse models. The results indicate that Car inhibition impaired male fertility due to altered sperm quality, specifically histone retention, which is correlated with an increased percentage of dying offspring in utero. The data highlighted interactions among Car, Akt, Foxo1, and histone acetylation. This study demonstrates that Car is crucial in germ cell homeostasis and male fertility. Further research on the Car signaling pathway is necessary to reveal unidentified causes of altered fertility and understand the harmful impact of environmental molecules on male fertility and offspring health.
Keywords: Foxo1; constitutive androstane receptor; repro‐toxicity; spermatogonia; xenobiotic.
© 2024 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








References
-
- Sharlip I. D., Jarow J. P., Belker A. M., Lipshultz L. I., Sigman M., Thomas A. J., Schlegel P. N., Howards S. S., Nehra A., Damewood M. D., Overstreet J. W., Sadovsky R., Fertil. Steril. 2002, 77, 873. - PubMed
-
- de Rooij D. G., Reproduction 2001, 121, 347. - PubMed
-
- Siklenka K., Erkek S., Godmann M., Lambrot R., McGraw S., Lafleur C., Cohen T., Xia J., Suderman M., Hallett M., Trasler J., Peters A. H. F. M., Kimmins S., Science 2015, 350, aab2006. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
