The role of ATP citrate lyase in myelin formation and maintenance
- PMID: 39318247
- PMCID: PMC11660526
- DOI: 10.1002/glia.24620
The role of ATP citrate lyase in myelin formation and maintenance
Abstract
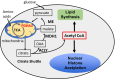
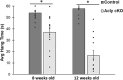
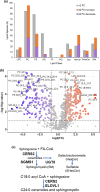
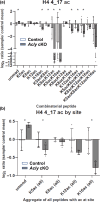
Formation of myelin by Schwann cells is tightly coupled to peripheral nervous system development and is important for neuronal function and long-term maintenance. Perturbation of myelin causes a number of specific disorders that are among the most prevalent diseases affecting the nervous system. Schwann cells synthesize myelin lipids de novo rather than relying on uptake of circulating lipids, yet one unresolved matter is how acetyl CoA, a central metabolite in lipid formation is generated during myelin formation and maintenance. Recent studies have shown that glucose-derived acetyl CoA itself is not required for myelination. However, the importance of mitochondrially-derived acetyl CoA has never been tested for myelination in vivo. Therefore, we have developed a Schwann cell-specific knockout of the ATP citrate lyase (Acly) gene to determine the importance of mitochondrial metabolism to supply acetyl CoA in nerve development. Intriguingly, the ACLY pathway is important for myelin maintenance rather than myelin formation. In addition, ACLY is required to maintain expression of a myelin-associated gene program and to inhibit activation of the latent Schwann cell injury program.
Keywords: Schwann; acetyl CoA; lipid; lipidomic; myelin.
© 2024 The Author(s). GLIA published by Wiley Periodicals LLC.
Figures




Similar articles
-
A hierarchical hepatic de novo lipogenesis substrate supply network utilizing pyruvate, acetate, and ketones.Cell Metab. 2025 Jan 7;37(1):255-273.e6. doi: 10.1016/j.cmet.2024.10.013. Epub 2024 Oct 28. Cell Metab. 2025. PMID: 39471817
-
ACSS2-mediated acetyl-CoA synthesis from acetate is necessary for human cytomegalovirus infection.Proc Natl Acad Sci U S A. 2017 Feb 21;114(8):E1528-E1535. doi: 10.1073/pnas.1614268114. Epub 2017 Feb 6. Proc Natl Acad Sci U S A. 2017. PMID: 28167750 Free PMC article.
-
Modulation of matrix metabolism by ATP-citrate lyase in articular chondrocytes.J Biol Chem. 2018 Aug 3;293(31):12259-12270. doi: 10.1074/jbc.RA118.002261. Epub 2018 Jun 21. J Biol Chem. 2018. PMID: 29929979 Free PMC article.
-
Exploring the Role of ATP-Citrate Lyase in the Immune System.Front Immunol. 2021 Feb 18;12:632526. doi: 10.3389/fimmu.2021.632526. eCollection 2021. Front Immunol. 2021. PMID: 33679780 Free PMC article. Review.
-
ATP citrate lyase: A central metabolic enzyme in cancer.Cancer Lett. 2020 Feb 28;471:125-134. doi: 10.1016/j.canlet.2019.12.010. Epub 2019 Dec 9. Cancer Lett. 2020. PMID: 31830561 Review.
References
-
- Arthur‐Farraj, P. J. , Latouche, M. , Wilton, D. K. , Quintes, S. , Chabrol, E. , Banerjee, A. , Woodhoo, A. , Jenkins, B. , Rahman, M. , Turmaine, M. , Wicher, G. K. , Mitter, R. , Greensmith, L. , Behrens, A. , Raivich, G. , Mirsky, R. , & Jessen, K. R. (2012). c‐Jun reprograms Schwann cells of injured nerves to generate a repair cell essential for regeneration. Neuron, 75(4), 633–647. 10.1016/j.neuron.2012.06.021 - DOI - PMC - PubMed
-
- Arthur‐Farraj, P. J. , Morgan, C. C. , Adamowicz, M. , Gomez‐Sanchez, J. A. , Fazal, S. V. , Beucher, A. , Razzaghi, B. , Mirsky, R. , Jessen, K. R. , & Aitman, T. J. (2017). Changes in the coding and non‐coding transcriptome and DNA methylome that define the Schwann cell repair phenotype after nerve injury. Cell Reports, 20(11), 2719–2734. 10.1016/j.celrep.2017.08.064 - DOI - PMC - PubMed
-
- Bai, Y. , Treins, C. , Volpi, V. G. , Scapin, C. , Ferri, C. , Mastrangelo, R. , Touvier, T. , Florio, F. , Bianchi, F. , Del Carro, U. , Baas, F. F. , Wang, D. , Miniou, P. , Guedat, P. , Shy, M. E. , & D'Antonio, M. (2022). Treatment with IFB‐088 improves neuropathy in CMT1A and CMT1B mice. Molecular Neurobiology, 59(7), 4159–4178. 10.1007/s12035-022-02838-y - DOI - PMC - PubMed
-
- Beirowski, B. , Gustin, J. , Armour, S. M. , Yamamoto, H. , Viader, A. , North, B. J. , Michán, S. , Baloh, R. H. , Golden, J. P. , Schmidt, R. E. , Sinclair, D. A. , Auwerx, J. , & Milbrandt, J. (2011). Sir‐two‐homolog 2 (Sirt2) modulates peripheral myelination through polarity protein Par‐3/atypical protein kinase C (aPKC) signaling. Proceedings of the National Academy of Sciences of the United States of America, 108(43), E952–E961. 10.1073/pnas.1104969108 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

