Integrative metagenomic and lipidomic analyses reveal alterations in children with obesity and after lifestyle intervention
- PMID: 39318384
- PMCID: PMC11420138
- DOI: 10.3389/fnut.2024.1423724
Integrative metagenomic and lipidomic analyses reveal alterations in children with obesity and after lifestyle intervention
Abstract
Background: Despite emerging evidence linking alterations in gut microbiota to childhood obesity, the metabolic mechanisms linking gut microbiota to the lipid profile during childhood obesity and weight loss remain poorly understood.
Methodology: In this study, children with obesity were treated with lifestyle weight loss therapy. Metagenomics association studies and serum untargeted lipidomics analyses were performed in children with obesity and healthy controls before and after weight loss.
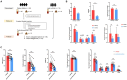
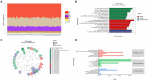
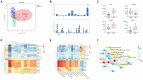
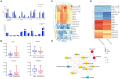
Main findings: We identified alterations in gut microbiota associated with childhood obesity, as well as variations in circulating metabolite concentrations. Children with obesity showed significant decreases in the levels of s-Rothia_kristinae and s-Enterobacter_roggenkampii, alongsige elevated levels of s-Clostridiales_bacterium_Marseille-P5551. Following weight loss, the levels of s-Streptococcus_infantarius and s-Leuconostoc_citreum increased by factors of 3.354 and 1.505, respectively, in comparison to their pre-weight loss levels. Correlation analyses indicated a significant positive relationship between ChE(2:0) levels and both with s-Lachnospiraceae_bacterium_TF09-5 and fasting glucose levels. CoQ8 levels were significantly negatively correlated with s-Rothia_kristinae and HOMA-IR.
Conclusion: We linked altered gut microbiota and serum lipid levels in children with obesity to clinical indicators, indicating a potential impact on glucose metabolism via lipids. This study contributes to understanding the mechanistic relationship between altered gut microbiota and childhood obesity and weight loss, suggesting gut microbiome as a promising target for intervention.
Clinical trial registration: https://www.chictr.org.cn/showproj.html?proj=178971, ChiCTR2300072179.
Keywords: childhood; lipidomic; metagenomics; obesity; weight loss.
Copyright © 2024 Yin, Liu, Xu, Li, Li, Qin, Zhang, Sun, Liu and Xiao.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Characterization of gut microbial and metabolite alterations in faeces of Goto Kakizaki rats using metagenomic and untargeted metabolomic approach.World J Diabetes. 2023 Mar 15;14(3):255-270. doi: 10.4239/wjd.v14.i3.255. World J Diabetes. 2023. PMID: 37035219 Free PMC article.
-
Effect of "maccog" TCM tea on improving glucolipid metabolism and gut microbiota in patients with type 2 diabetes in community.Front Endocrinol (Lausanne). 2023 Mar 8;14:1134877. doi: 10.3389/fendo.2023.1134877. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 36967788 Free PMC article.
-
Canagliflozin alters the gut, oral, and ocular surface microbiota of patients with type 2 diabetes mellitus.Front Endocrinol (Lausanne). 2023 Oct 5;14:1256292. doi: 10.3389/fendo.2023.1256292. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37867512 Free PMC article.
-
Gut Microbiota and Obesity in Adults and Children: The State of the Art.Front Pediatr. 2021 Mar 19;9:657020. doi: 10.3389/fped.2021.657020. eCollection 2021. Front Pediatr. 2021. PMID: 33816411 Free PMC article. Review.
-
The Gut Microbiome Profile in Obesity: A Systematic Review.Int J Endocrinol. 2018 Mar 22;2018:4095789. doi: 10.1155/2018/4095789. eCollection 2018. Int J Endocrinol. 2018. PMID: 29849617 Free PMC article. Review.
Cited by
-
Lipidomic signatures linked to gut microbiota alterations in children and adolescents with type 2 diabetes mellitus and metabolic syndrome.Sci Rep. 2025 Jun 3;15(1):19427. doi: 10.1038/s41598-025-04343-3. Sci Rep. 2025. PMID: 40461716 Free PMC article.
References
-
- Phelps NH, Singleton RK, Zhou B, Heap RA, Mishra A, Bennett JE, et al. . Worldwide trends in underweight and obesity from 1990 to 2022: a pooled analysis of 3663 population-representative studies with 222 million children, adolescents, and adults. Lancet. (2024) 403:1027–50. doi: 10.1016/S0140-6736(23)02750-2, PMID: - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

