Harnessing biocatalysis as a green tool in antibiotic synthesis and discovery
- PMID: 39318457
- PMCID: PMC11420778
- DOI: 10.1039/d4ra04824e
Harnessing biocatalysis as a green tool in antibiotic synthesis and discovery
Abstract
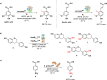
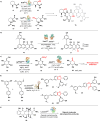
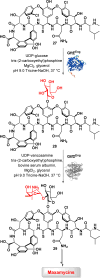
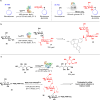
Biocatalysis offers a sustainable approach to drug synthesis, leveraging the high selectivity and efficiency of enzymes. This review explores the application of biocatalysis in the early-stage synthesis of antimicrobial compounds, emphasizing its advantages over traditional chemical methods. We discuss various biocatalysts, including enzymes and whole-cell systems, and their role in the selective functionalization and preparation of antimicrobials and antibacterial building blocks. The review underscores the potential of biocatalysis to advance the development of new antibiotics and suggests directions and potential applications of enzymes in drug development.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures

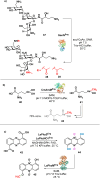





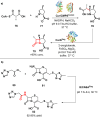
References
-
- Gaynes R. Emerg. Infect. Dis. 2017;23:849–853.
Publication types
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

