Crystallin β-b2 promotes retinal ganglion cell protection in experimental autoimmune uveoretinitis
- PMID: 39318470
- PMCID: PMC11419989
- DOI: 10.3389/fncel.2024.1379540
Crystallin β-b2 promotes retinal ganglion cell protection in experimental autoimmune uveoretinitis
Abstract
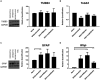
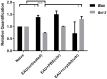
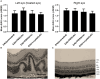
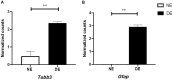
Crystallin βb2 (crybb2) is upregulated in regenerating retinas and in various pathological conditions of the retina, including uveoretinitis. However, the role of crybb2 in this disease is largely unknown. Therefore, we used recombinant crybb2 (rcrybb2) as intravitreal treatment of B10.RIII mice prior to immunization with human interphotoreceptor retinoid-binding protein peptide 161-180 (hIRBPp161-180) in complete Freund's adjuvant (CFA) and concomitant injection of pertussis toxin (PTX) to induce experimental autoimmune uveoretinitis (EAU). In naïve mice, more beta III-tubulin (TUBB3) + and RNA-binding protein with multiple splicing (RBPMS) + cells were found in the ganglion cell layer of the retina than in EAU eyes, suggesting a loss of retinal ganglion cells (RGC) during the development of EAU. At the same time, the number of glial fibrillary acidic protein (GFAP) + cells increased in EAU eyes. RGCs were better protected in EAU eyes treated with rcrybb2, while the number of GFAP+ cells decreased. However, in retinal flatmounts, both retinal ganglion cells and retinal endothelial cells stained positive for TUBB3, indicating that TUBB3 is present in naïve B10.RIII mouse eyes not exclusive to RGCs. A significant decline in the number of RBPMS-positive retinal ganglion cells was observed in retinal flatmounts from EAU retinas in comparison to naïve retinas or EAU retinas with intravitreal rcrybb2 treatment. Whereas no significant decrease in TUBB3 levels was detected using Western blot and RT-qPCR, GFAP level, as a marker for astrocytes, increased in EAU mice compared to naïve mice. Level of Bax and Bcl2 in the retina was altered by treatment, suggesting better cell survival and inhibition of apoptosis. Furthermore, our histologic observations of the eyes showed no change in the incidence and severity of EAU, nor was the immune response affected by intravitreal rcrybb2 treatment. Taken together, these results suggest that intravitreal injection of rcrybb2 reduces retinal RGC death during the course of EAU, independent of local or systemic autoimmune responses. In the future, treating posterior uveitis with rcrybb2 to protect RGCs may offer a promising novel therapeutic strategy.
Keywords: apoptosis; crystallin β-b2; experimental autoimmune uveitis; neuroprotection; retinal ganglion cell.
Copyright © 2024 Bauer, Böhm, Wu, Wang, Jalilvand, Busch, Kasper, Brockhaus, Wildschütz, Melkonyan, Laffer, Meyer Zu Hörste, Heiligenhaus and Thanos.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
Kinetic changes in microglia-related retinal transcripts in experimental autoimmune uveoretinitis (EAU) of B10.RIII mice.J Neuroinflammation. 2025 Feb 10;22(1):37. doi: 10.1186/s12974-025-03358-x. J Neuroinflammation. 2025. PMID: 39930455 Free PMC article.
-
Visual stimulation and brain-derived neurotrophic factor (BDNF) have protective effects in experimental autoimmune uveoretinitis.Life Sci. 2024 Oct 15;355:122996. doi: 10.1016/j.lfs.2024.122996. Epub 2024 Aug 20. Life Sci. 2024. PMID: 39173995
-
The requirement for pertussis to induce EAU is strain-dependent: B10.RIII, but not B10.A mice, develop EAU and Th1 responses to IRBP without pertussis treatment.Invest Ophthalmol Vis Sci. 1999 Nov;40(12):2898-905. Invest Ophthalmol Vis Sci. 1999. PMID: 10549650
-
[Translational research with experimental autoimmune uveoretinitis (EAU)].Nippon Ganka Gakkai Zasshi. 2007 Mar;111(3):137-58; discussion 159. Nippon Ganka Gakkai Zasshi. 2007. PMID: 17402560 Review. Japanese.
-
Experimental autoimmune uveoretinitis in the rat and mouse.Curr Protoc Immunol. 2003 May;Chapter 15:15.6.1-15.6.20. doi: 10.1002/0471142735.im1506s53. Curr Protoc Immunol. 2003. PMID: 18432901 Review.
Cited by
-
Analysis of mouse lens morphological and proteomic abnormalities following depletion of βB3-crystallin.bioRxiv [Preprint]. 2024 Dec 30:2024.12.30.630781. doi: 10.1101/2024.12.30.630781. bioRxiv. 2024. Update in: Exp Eye Res. 2025 Aug 19;260:110587. doi: 10.1016/j.exer.2025.110587. PMID: 39803551 Free PMC article. Updated. Preprint.
-
Kinetic changes in microglia-related retinal transcripts in experimental autoimmune uveoretinitis (EAU) of B10.RIII mice.J Neuroinflammation. 2025 Feb 10;22(1):37. doi: 10.1186/s12974-025-03358-x. J Neuroinflammation. 2025. PMID: 39930455 Free PMC article.
-
Retina-Targeted 17β-Estradiol by the DHED Prodrug Rescues Visual Function and Actuates Neuroprotective Protein Networks After Optic Nerve Crush in a Rat Model of Surgical Menopause.Int J Mol Sci. 2025 Feb 21;26(5):1846. doi: 10.3390/ijms26051846. Int J Mol Sci. 2025. PMID: 40076480 Free PMC article.
-
Polyphenol-based self-assembled nanoparticles treating uveitis by inflammation-oxidative stress suppression.Mater Today Bio. 2025 Jul 4;33:102052. doi: 10.1016/j.mtbio.2025.102052. eCollection 2025 Aug. Mater Today Bio. 2025. PMID: 40688683 Free PMC article.
References
-
- Barnett N. L., Osborne N. N. (1995). Redistribution of GABA immunoreactivity following central retinal artery occlusion. Brain Res. 677, 337–340. - PubMed
-
- Bauer D., Wasmuth S., Hennig M., Baehler H., Steuhl K. P., Heiligenhaus A. (2009). Amniotic membrane transplantation induces apoptosis in T lymphocytes in murine corneas with experimental herpetic stromal keratitis. Invest. Ophthalmol. Vis. Sci. 50, 3188–3198. doi: 10.1167/iovs.08-3041, PMID: - DOI - PubMed
-
- Block F., Schwarz M., Sontag K. H. (1992). Retinal ischemia induced by occlusion of both common carotid arteries in rats as demonstrated by electroretinography. Neurosci. Lett. 144, 124–126. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

