Characteristics and real-world medication persistence of people living with HIV treated with DTG/3TC or BIC/FTC/TAF: a hospital claims database study in Japan
- PMID: 39318599
- PMCID: PMC11420020
- DOI: 10.3389/fmed.2024.1329922
Characteristics and real-world medication persistence of people living with HIV treated with DTG/3TC or BIC/FTC/TAF: a hospital claims database study in Japan
Erratum in
-
Corrigendum: Characteristics and real-world medication persistence of people living with HIV treated with DTG/3TC or BIC/FTC/TAF: a hospital claims database study in Japan.Front Med (Lausanne). 2025 Feb 10;12:1555353. doi: 10.3389/fmed.2025.1555353. eCollection 2025. Front Med (Lausanne). 2025. PMID: 39995687 Free PMC article.
Abstract
Background: As the life expectancy of people living with human immunodeficiency virus (HIV) (PLWH) has improved, chronic disease burden and polypharmacy have increased in PLWH. Simplification of the antiretroviral therapy (ART) regimen for PLWH has become crucial. The real-world treatment patterns and medication persistence of the 2-drug single-tablet regimen (STR), dolutegravir/lamivudine (DTG/3TC), compared to bictegravir/emtricitabine/tenofovir alafenamide (BIC/FTC/TAF) prescribed were investigated.
Methods: This retrospective, database study extracted data from a hospital-based medical claims database in Japan. The changes in ART distributions by year during the identification period between January 1, 2018 and December 31, 2021 were observed. Patients with disease record of HIV-1 infection and prescribed DTG/3TC or BIC/FTC/TAF as the first prescription of STR during the identification period were divided into two cohorts; DTG/3TC cohort and BIC/FTC/TAF cohort, respectively. Patient without medication records more than 3 months and no future data more than 6 months were excluded. Patients' characteristics were compared between the DTG/3TC cohort and the BIC/FTC/TAF cohort by Mantel-Haenszel test to adjust for age. Medication persistence was compared between the two cohorts by evaluating the continuation rates using Kaplan-Meier methods, using the log-rank test to assess the difference between the Kaplan-Meier curves. The median time-to-first prescription was compared between the two cohorts by Kaplan-Meier methods.
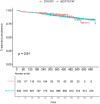
Results: Prescriptions of DTG/3TC and BIC/FTC/TAF increased steadily from 2019 to 2021 after the release year of each STR. There was no significant difference in the time-to-first prescription (p = 0.3). A total of 959 patients were included, with 120 patients and 839 patients on DTG/3TC and BIC/FTC/TAF, respectively. The proportion of dyslipidemia at baseline was significantly higher in the DTG/3TC cohort than in the BIC/FTC/TAF cohort after adjusting for mean age (p = 0.002). There was no significant difference in medication persistence between the two cohorts (p = 0.91).
Conclusion: This study showed that DTG/3TC was likely to be selected for elderly patients and those with chronic disease in real-world clinical practice, which seems in accordance with the treatment strategy recommended by guidelines. Comparable medication persistence was observed with both regimens, aligning with findings from other countries. The 2-drug single-tablet regimen DTG/3TC may be an important ART regimen for PLWH with multiple morbidities and polypharmacy in an aging society. Due to the limitations of the database, further research to assess viral loads, emergence of resistance and adverse events will be encouraged.
Keywords: HIV; aging society; antiretroviral therapy; characteristics; medication persistence; real-world; single-tablet regimen; treatment pattern.
Copyright © 2024 Kanamori, Aoki, Kanazawa, Yuda, Makino, Ohata, Fukui, Mori, Yokokawa and Naito.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Comparing Real-World Healthcare Costs Associated with Single-Tablet Regimens for HIV-1: The 2-Drug Regimen Dolutegravir/Lamivudine vs. Standard 3- or 4-Drug Regimens.Infect Dis Ther. 2023 Aug;12(8):2117-2133. doi: 10.1007/s40121-023-00848-4. Epub 2023 Aug 8. Infect Dis Ther. 2023. PMID: 37552426 Free PMC article.
-
[Translated article] Real-world persistence with dolutegravir/lamivudine versus bictegravir/emtricitabine/tenofovir-alafenamide among persons with HIV.Farm Hosp. 2024 Jul-Aug;48(4):T171-T175. doi: 10.1016/j.farma.2024.02.016. Epub 2024 May 27. Farm Hosp. 2024. PMID: 38806363 English, Spanish.
-
Treatment persistence of bictegravir/emtricitabine/tenofovir alafenamide and efavirenz + lamivudine + tenofovir disoproxil among HIV-1 patients newly starting treatment in Hunan Province in China.BMC Infect Dis. 2023 Jun 12;23(1):396. doi: 10.1186/s12879-023-08359-w. BMC Infect Dis. 2023. PMID: 37308847 Free PMC article.
-
An indirect comparison of 144-week efficacy, safety, and tolerability of dolutegravir plus lamivudine and second-generation integrase inhibitor-based, 3-drug, single-tablet regimens in therapy-naive people with HIV-1.AIDS Res Ther. 2023 Mar 22;20(1):17. doi: 10.1186/s12981-023-00507-1. AIDS Res Ther. 2023. PMID: 36949442 Free PMC article.
-
Comparison of the design and methodology of Phase 3 clinical trials of bictegravir/emtricitabine/tenofovir alafenamide (BIC/FTC/TAF) and dolutegravir-based dual therapy (DTG) in HIV: a systematic review of the literature.Expert Rev Anti Infect Ther. 2023 Jan;21(1):65-76. doi: 10.1080/14787210.2023.2149490. Epub 2022 Nov 27. Expert Rev Anti Infect Ther. 2023. PMID: 36399521
References
-
- Samji H, Cescon A, Hogg RS, Modur SP, Althoff KN, Buchacz K, et al. . North American AIDS cohort collaboration on research and design (NA-ACCORD) of IeDEA. Closing the gap: increases in life expectancy among treated HIV-positive individuals in the United States and Canada. PLoS One. (2013) 8:e81355. doi: 10.1371/journal.pone.0081355, PMID: - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

