Effects of once-weekly subcutaneous retatrutide on weight and metabolic markers: A systematic review and meta-analysis of randomized controlled trials
- PMID: 39318607
- PMCID: PMC11420505
- DOI: 10.1016/j.metop.2024.100321
Effects of once-weekly subcutaneous retatrutide on weight and metabolic markers: A systematic review and meta-analysis of randomized controlled trials
Abstract
Aim: To assess the effects of once-weekly subcutaneous retatrutide on weight and metabolic markers and the occurrence of side effects in patients with overweight, obesity and/or type 2 diabetes (T2D).
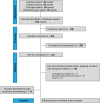
Methods: PubMed, Embase, Cochrane Library, and ClinicalTrials.gov databases were systematically searched for placebo-controlled, randomized clinical trials (RCTs) published up until February 23, 2024. Weighted mean differences (WMDs) for continuous outcomes and risk ratios (RRs) for binary endpoints were computed, with 95 % confidence intervals (CIs).
Results: A total of three studies were included, comprising 640 patients, of whom 510 were prescribed retatrutide. Compared with placebo, retatrutide significantly reduced body weight (WMD -10.66 kg; 95 % CI -17.63, -3.69), body mass index (WMD -4.53 kg/m2; 95 % CI -7.51, -1.55), and waist circumference (WMD -6.61 cm; 95 % CI -13.17, -0.05). In addition, retatrutide significantly increased the proportion of patients who achieved a weight reduction of ≥5 % (RR 2.92; 95 % CI 2.17-3.93), ≥10 % (RR 9.32; 95 % CI 4.56-19.06), ≥15 % (RR 18.40; 95 % CI 6.00-56.42), and ≥20 % (RR 16.61; 95 % CI 4.17-66.12).
Conclusions: In this meta-analysis, the use of once-weekly subcutaneous retatrutide was associated with a significant reduction in body weight and improvement of metabolic markers in patients with overweight, obesity and/or T2D, compared with placebo, with an increase in non-severe gastrointestinal and hypersensitivity adverse events. Phase 3 RCTs are expected to shed further light on the efficacy and safety of once-weekly subcutaneous retatrutide over the long term.
Keywords: Body weight; Metabolism; Obesity; Retatrutide.
© 2024 The Authors.
Figures



References
-
- World Obesity Federation World obesity atlas 2023. 2023. https://data.worldobesity.org/publications/WOF-Obesity-Atlas-V5.pdf
Publication types
LinkOut - more resources
Full Text Sources

