Predicting Choroidal Nevus Transformation to Melanoma Using Machine Learning
- PMID: 39318711
- PMCID: PMC11421339
- DOI: 10.1016/j.xops.2024.100584
Predicting Choroidal Nevus Transformation to Melanoma Using Machine Learning
Abstract
Purpose: To develop and validate machine learning (ML) models to predict choroidal nevus transformation to melanoma based on multimodal imaging at initial presentation.
Design: Retrospective multicenter study.
Participants: Patients diagnosed with choroidal nevus on the Ocular Oncology Service at Wills Eye Hospital (2007-2017) or Mayo Clinic Rochester (2015-2023).
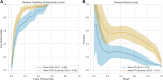
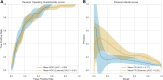
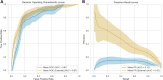
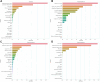
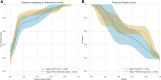
Methods: Multimodal imaging was obtained, including fundus photography, fundus autofluorescence, spectral domain OCT, and B-scan ultrasonography. Machine learning models were created (XGBoost, LGBM, Random Forest, Extra Tree) and optimized for area under receiver operating characteristic curve (AUROC). The Wills Eye Hospital cohort was used for training and testing (80% training-20% testing) with fivefold cross validation. The Mayo Clinic cohort provided external validation. Model performance was characterized by AUROC and area under precision-recall curve (AUPRC). Models were interrogated using SHapley Additive exPlanations (SHAP) to identify the features most predictive of conversion from nevus to melanoma. Differences in AUROC and AUPRC between models were tested using 10 000 bootstrap samples with replacement and results.
Main outcome measures: Area under receiver operating curve and AUPRC for each ML model.
Results: There were 2870 nevi included in the study, with conversion to melanoma confirmed in 128 cases. Simple AI Nevus Transformation System (SAINTS; XGBoost) was the top-performing model in the test cohort [pooled AUROC 0.864 (95% confidence interval (CI): 0.864-0.865), pooled AUPRC 0.244 (95% CI: 0.243-0.246)] and in the external validation cohort [pooled AUROC 0.931 (95% CI: 0.930-0.931), pooled AUPRC 0.533 (95% CI: 0.531-0.535)]. Other models also had good discriminative performance: LGBM (test set pooled AUROC 0.831, validation set pooled AUROC 0.815), Random Forest (test set pooled AUROC 0.812, validation set pooled AUROC 0.866), and Extra Tree (test set pooled AUROC 0.826, validation set pooled AUROC 0.915). A model including only nevi with at least 5 years of follow-up demonstrated the best performance in AUPRC (test: pooled 0.592 (95% CI: 0.590-0.594); validation: pooled 0.656 [95% CI: 0.655-0.657]). The top 5 features in SAINTS by SHAP values were: tumor thickness, largest tumor basal diameter, tumor shape, distance to optic nerve, and subretinal fluid extent.
Conclusions: We demonstrate accuracy and generalizability of a ML model for predicting choroidal nevus transformation to melanoma based on multimodal imaging.
Financial disclosures: Proprietary or commercial disclosure may be found in the Footnotes and Disclosures at the end of this article.
Keywords: Artificial Intelligence; Choroidal melanoma; Choroidal nevus; Machine learning; Ocular oncology.
© 2024 by the American Academy of Ophthalmology.
Figures

References
-
- Shields J.A., Shields C.L. 3 ed. Wolters Kluwer Health; Philadelphia, PA: 2016. Intraocular Tumors: An Atlas and Textbook.
-
- Shields C.L., Dalvin L.A., Ancona-Lezama D., et al. Choroidal nevus imaging features in 3,806 cases and risk factors for transformation into melanoma in 2,355 cases: the 2020 Taylor R. Smith and Victor T. Curtin Lecture. Retina. 2019;39:1840–1851. - PubMed
-
- Kwong J.C.C., Khondker A., Meng E., et al. Development, multi-institutional external validation, and algorithmic audit of an artificial intelligence-based Side-specific Extra-Prostatic Extension Risk Assessment tool (SEPERA) for patients undergoing radical prostatectomy: a retrospective cohort study. Lancet Digit Health. 2023;5:e435–e445. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

