An overview of immune checkpoint inhibitor toxicities in bladder cancer
- PMID: 39318722
- PMCID: PMC11420502
- DOI: 10.1016/j.toxrep.2024.101732
An overview of immune checkpoint inhibitor toxicities in bladder cancer
Abstract
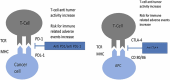
Bladder cancer is the tenth most prevalent malignancy worldwide, with a significant mortality burden. Urothelial carcinoma (UC) is the most common histological subtype, and treatment options are guided by whether the disease is muscle-invasive (MIBC) or non-muscle-invasive (NMIBC), with subsequent risk group stratification. The growing popularity of immune checkpoint inhibitors (ICIs) to treat MIBC and NMIBC as either monotherapy or combined with intravesical agents, may radically change the treatment paradigm of UC. Current treatments for NMBIC includes intravesical chemotherapy after trans-urethral resection of the bladder tumour, intravesical bacillus Calmette-Guerin (BCG) or radical cystectomy. Cisplatin-based chemotherapy is widely regarded as the first-line treatment for metastatic UC due to its beneficial response and survival rates when compared to alternative therapies. However, up to 70 % of metastatic UC patients are ineligible, and the prognosis of these patients remains poor, with a median survival of 13-16 months. For NMIBC and MIBC, ICIs provide a promising alternative for cisplatin-ineligible patients. In UC, ICIs including atezolizumab, nivolumab, avelumab, and pembrolizumab are Food and Drug Administration (FDA)-approved for monotherapy, and have demonstrated promising results, particularly in those who cannot receive cisplatin-based chemotherapy, and as a second-line treatment option for recurrent UC following platinum-based chemotherapy. It is important to consider that some patients may experience adverse events (AEs) with limited clinical benefit. Infusion-related reactions and immune-mediated AEs (imAEs) such as colitis, endocrinopathies, hepatitis, pneumonitis, interstitial lung disease, renal dysfunction, nephritis, cutaneous and neurological toxicities must be monitored for. Currently, there is no clear consensus on the role of a 'two-year stopping rule' in reducing the risk of imAEs, with further research on the optimal treatment duration of ICIs required. With increased ICI use, vigilance regarding their side effects is imperative. This review aims to provide an updated overview of ICI toxicities in bladder cancer, to assist clinicians in their therapeutic decision-making, with consideration of patient characteristics and the clinical context.
Keywords: Bladder Cancer; CTLA-4; Immune Checkpoint Inhibitor; PD-1; PD-L1; Toxicity.
© 2024 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
Similar articles
-
Immune checkpoint inhibitors for urothelial carcinoma.Investig Clin Urol. 2018 Sep;59(5):285-296. doi: 10.4111/icu.2018.59.5.285. Epub 2018 Aug 31. Investig Clin Urol. 2018. PMID: 30182073 Free PMC article. Review.
-
Single or combined immune checkpoint inhibitors compared to first-line platinum-based chemotherapy with or without bevacizumab for people with advanced non-small cell lung cancer.Cochrane Database Syst Rev. 2020 Dec 14;12(12):CD013257. doi: 10.1002/14651858.CD013257.pub2. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2021 Apr 30;4:CD013257. doi: 10.1002/14651858.CD013257.pub3. PMID: 33316104 Free PMC article. Updated.
-
Single or combined immune checkpoint inhibitors compared to first-line platinum-based chemotherapy with or without bevacizumab for people with advanced non-small cell lung cancer.Cochrane Database Syst Rev. 2021 Apr 30;4(4):CD013257. doi: 10.1002/14651858.CD013257.pub3. Cochrane Database Syst Rev. 2021. PMID: 33930176 Free PMC article.
-
A Multi-Gene Signature of Non-Muscle-Invasive Bladder Cancer Identifies Patients Who Respond to Immunotherapies Including Bacillus Calmette-Guérin and Immune Checkpoint Inhibitors.Int J Mol Sci. 2024 Mar 28;25(7):3800. doi: 10.3390/ijms25073800. Int J Mol Sci. 2024. PMID: 38612609 Free PMC article.
-
Profile of pembrolizumab in the treatment of patients with unresectable or metastatic urothelial carcinoma.Cancer Manag Res. 2019 May 15;11:4519-4528. doi: 10.2147/CMAR.S167708. eCollection 2019. Cancer Manag Res. 2019. PMID: 31191013 Free PMC article. Review.
Cited by
-
State of the art of adjuvant immunotherapy in urothelial cancer: New developments and upcoming changes.Hum Vaccin Immunother. 2025 Dec;21(1):2440165. doi: 10.1080/21645515.2024.2440165. Epub 2024 Dec 19. Hum Vaccin Immunother. 2025. PMID: 39701156 Free PMC article. Review.
-
circICMT upregulates and suppresses the malignant behavior of bladder cancer.Transl Oncol. 2025 Feb;52:102262. doi: 10.1016/j.tranon.2024.102262. Epub 2024 Dec 28. Transl Oncol. 2025. PMID: 39733742 Free PMC article.
-
Efficacy and safety of atezolizumab in the treatment of urothelial carcinoma: a systematic review and meta-analysis.World J Surg Oncol. 2025 Apr 9;23(1):133. doi: 10.1186/s12957-025-03795-1. World J Surg Oncol. 2025. PMID: 40205427 Free PMC article.
-
An Adverse Double-Hit by Pembrolizumab: A Case Report of Bullous Pemphigoid and Pneumonitis.J Med Cases. 2025 Feb;16(2):69-76. doi: 10.14740/jmc5089. Epub 2025 Feb 2. J Med Cases. 2025. PMID: 39935545 Free PMC article.
-
Development and Validation of a Competitive Risk Model in Elderly Patients with Transitional Cell Bladder Carcinoma.Med Sci Monit. 2025 Jan 29;31:e946332. doi: 10.12659/MSM.946332. Med Sci Monit. 2025. PMID: 39876530 Free PMC article.
References
-
- Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021;71(3):209–249. - PubMed
-
- Powles T., Bellmunt J., Comperat E., Santis M.D., Huddart R., Loriot Y., et al. Bladder cancer: ESMO clinical practice guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2022;33(3):244–258. - PubMed
-
- Uccello M., Adeleke S., Moschetta M., Ghose A., Boussios S. Immunotherapy for advanced urothelial carcinoma (UC): rational and current evidence. Ann. Palliat. Med. 2023;12(6):1345–1354. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

