A New Immunogenic Structure of Polyepitopic Fusion against Leishmania major: In Silico Study
- PMID: 39318820
- PMCID: PMC11417980
- DOI: 10.18502/ijpa.v19i3.16387
A New Immunogenic Structure of Polyepitopic Fusion against Leishmania major: In Silico Study
Abstract
Background: The lack of complete protection against leishmaniasis and the challenges of anti-leishmaniasis drug treatment have made the treatment process more difficult. This study aimed to develop a new strategy for preparing a vaccine against cutaneous leishmaniasis using some of the antigenic proteins of the Leishmania parasite.
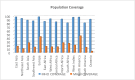
Methods: This study was carried out in 2022 at Shahid Chamran University of Ahvaz, Ahvaz, Iran. After preparing suitable epitopes of the Leishmania parasite and examining their antiparasitic properties, the process of making a fusion vaccine was performed and with the help of various bioinformatics tools, physicochemical and structural properties as well as immunological and simulation properties were studied and finally optimized. Construction and cloning were performed in the E.coli K12 system and finally, the docking process was performed with Toll-like receptors (TLRs), major histocompatibility complex I (MHC-I), and MHC-II receptors. With the help of selected epitopes of the Leishmania parasite, which had a high percentage of population coverage, a stable, antigenic, and non-allergenic chimeric vaccine was predicted.
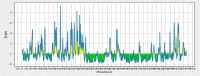
Results: The results of the structural analysis of the TLR5\vaccine complex and simulation of its molecular dynamics showed a sufficiently stable binding. It also showed good potential for stimulation and production of active B cells and memory, as well as the potential for CD8+ T, CD4+ T cell production and development of Th2 and Th1-induced immune responses.
Conclusion: Computational results showed that the designed immunogenic structure has the potential to adequately stimulate cellular and humoral immune responses against Leishmania parasitic disease. As a result of evaluating the effectiveness of the candidate vaccine through in vivo and in vitro immunological tests, it can be suggested as a vaccine against Leishmania major.
Keywords: Bioinformatics; Chimeric vaccine; Immunogenicity; Leishmania.
Copyright © 2024 Pirmoradi et al. Published by Tehran University of Medical Sciences.
Conflict of interest statement
Conflict of Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures









Similar articles
-
Bioinformatics analyses of immunogenic T-cell epitopes of LeIF and PpSP15 proteins from Leishmania major and sand fly saliva used as model antigens for the design of a multi-epitope vaccine to control leishmaniasis.Infect Genet Evol. 2020 Jun;80:104189. doi: 10.1016/j.meegid.2020.104189. Epub 2020 Jan 10. Infect Genet Evol. 2020. PMID: 31931259
-
An immunoinformatic approach driven by experimental proteomics: in silico design of a subunit candidate vaccine targeting secretory proteins of Leishmania donovani amastigotes.Parasit Vectors. 2020 Apr 15;13(1):196. doi: 10.1186/s13071-020-04064-8. Parasit Vectors. 2020. PMID: 32295617 Free PMC article.
-
Exploring membrane proteins of Leishmania major to design a new multi-epitope vaccine using immunoinformatics approach.Eur J Pharm Sci. 2020 Sep 1;152:105423. doi: 10.1016/j.ejps.2020.105423. Epub 2020 Jun 10. Eur J Pharm Sci. 2020. PMID: 32534195
-
Vaccinomics-based next-generation multi-epitope chimeric vaccine models prediction against Leishmania tropica - a hierarchical subtractive proteomics and immunoinformatics approach.Front Immunol. 2023 Sep 15;14:1259612. doi: 10.3389/fimmu.2023.1259612. eCollection 2023. Front Immunol. 2023. PMID: 37781384 Free PMC article.
-
[Immunopathology of American tegumentary leishmaniasis].Acta Cient Venez. 1998;49(1):42-56. Acta Cient Venez. 1998. PMID: 10205916 Review. Spanish.
Cited by
-
Comment on "A New Immunogenic Structure of Polyepitopic Fusion against Leishmania major: In Silico Study".Iran J Parasitol. 2024 Oct-Dec;19(4):515-516. doi: 10.18502/ijpa.v19i4.17175. Iran J Parasitol. 2024. PMID: 39735844 Free PMC article. No abstract available.
References
-
- Polonio T, Efferth T. Leishmaniasis: drug resistance and natural products. Int J Mol Med. 2008; 22(3): 277–286. - PubMed
-
- Engwerda CR, Matlashewski G. Development of Leishmania vaccines in the era of visceral leishmaniasis elimination. Trans R Soc Trop Med Hyg. 2015; 109(7):423–4. - PubMed
-
- Vakili B, Eslami M, Hatam GR, et al. Immunoinformatics-aided design of a potential multi-epitope peptide vaccine against Leishmania infantum. Int J Biol Macromol. 2018; 120(Pt A): 1127–1139. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
