Efficacy of Corticosteroids for Sore Throat Management in Adults: A Systematic Review and Meta-Analysis of Randomized Trials
- PMID: 39318911
- PMCID: PMC11421831
- DOI: 10.7759/cureus.67740
Efficacy of Corticosteroids for Sore Throat Management in Adults: A Systematic Review and Meta-Analysis of Randomized Trials
Abstract
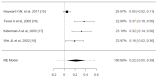
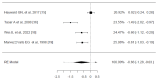
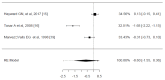
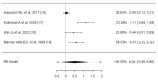
Sore throat (acute pharyngitis) is among the most common complaints among adults and is a reason for seeking healthcare globally. Antibiotics are widely used among patients with infectious sore throat. Previous research has indicated that corticosteroids could offer an alternative symptomatic treatment for sore throats. To estimate the corticosteroid efficacy as an additional therapy for sore throat adult patients, the literature search included PubMed, Medline, OVID, Cochrane CENTRAL, and Scopus for articles published until July 1st, 2024. The outcomes included the onset of pain relief (average time), complete resolution of pain (average time), absolute reduction of pain at 24 and 48 hours, requirement of antibiotics, and adverse effects related to treatment. Standardized mean difference (SMD) and risk difference were used to report numerical and dichotomous results. Five studies were included. Among the five included studies, corticosteroids showed significant effectiveness in resolving pain at 24 hours (average risk difference: 0.2200, 95% CI: 0.0500 to 0.3899, p = 0.0112) but with notable heterogeneity (I² = 82.4255%). At 48 hours, the benefit was not statistically significant (average risk difference: 0.4063, 95% CI: -0.1857 to 0.9984, p = 0.1786, I² = 98.9219%). Corticosteroids also decreased the average time to onset of pain relief (average SMD: -0.6590, 95% CI: -1.2857 to -0.0323, p = 0.0393, I² = 89.7914%), although with high heterogeneity. Other findings indicated a possible reduction in antibiotic use and fewer days missed from work. Adverse effects were minimal and occurred at similar rates in both corticosteroid and placebo arms. Corticosteroids can decrease pain intensity and duration in adults with acute sore throats. However, significant heterogeneity among studies and methodological limitations render the overall evidence inconclusive. While some studies noted reduced antibiotic use and lower symptom recurrence, high-quality RCTs are needed to address these limitations and provide more definitive guidelines for corticosteroid use in treating acute pharyngitis.
Keywords: clinical trials; corticosteroids; meta-analysis; pharyngitis; sore throat.
Copyright © 2024, Alqahtani et al.
Conflict of interest statement
Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following: Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work. Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work. Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
Figures
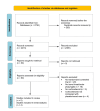



Similar articles
-
Oral corticosteroid use for clinical and cost-effective symptom relief of sore throat: study protocol for a randomized controlled trial.Trials. 2014 Sep 18;15:365. doi: 10.1186/1745-6215-15-365. Trials. 2014. PMID: 25238785 Free PMC article. Clinical Trial.
-
Tonsillectomy compared with conservative management in patients over 16 years with recurrent sore throat: the NATTINA RCT and economic evaluation.Health Technol Assess. 2023 Dec;27(31):1-195. doi: 10.3310/YKUR3660. Health Technol Assess. 2023. PMID: 38204203 Free PMC article. Clinical Trial.
-
Aerosolized corticosteroids to prevent postoperative sore throat in adults: A systematic review and meta-analysis.Acta Anaesthesiol Scand. 2019 Mar;63(3):282-291. doi: 10.1111/aas.13275. Epub 2018 Oct 14. Acta Anaesthesiol Scand. 2019. PMID: 30318587
-
Efficacy and safety of rapid tests to guide antibiotic prescriptions for sore throat.Cochrane Database Syst Rev. 2020 Jun 4;6(6):CD012431. doi: 10.1002/14651858.CD012431.pub2. Cochrane Database Syst Rev. 2020. PMID: 32497279 Free PMC article.
-
Different antibiotic treatments for group A streptococcal pharyngitis.Cochrane Database Syst Rev. 2021 Mar 17;3(3):CD004406. doi: 10.1002/14651858.CD004406.pub5. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2023 Nov 15;11:CD004406. doi: 10.1002/14651858.CD004406.pub6. PMID: 33728634 Free PMC article. Updated.
References
-
- Public knowledge and practice of sore throat management among visitors of primary care clinic in Riyadh, Saudi Arabia. Aloofy TA, Al-Ansary L, Mokhlis LG. J Adv Health Med Sci. 2017;3:1–8.
-
- Worldwide comparison of treatment guidelines for sore throat. Coutinho G, Duerden M, Sessa A, Caretta‐Barradas S, Altiner A. Int J Clin Pract. 2021;75:0.
-
- Incidence of hospital admissions due to adverse drug reactions in France: the EMIR study. Bénard-Laribière A, Miremont-Salamé G, Pérault-Pochat MC, Noize P, Haramburu F. Fundam Clin Pharmacol. 2015;29:106–111. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
