Adverse Events Associated With 3 Vitrectomy Platforms Reported to the US FDA MAUDE Database
- PMID: 39318983
- PMCID: PMC11418686
- DOI: 10.1177/24741264241264356
Adverse Events Associated With 3 Vitrectomy Platforms Reported to the US FDA MAUDE Database
Abstract
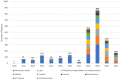
Purpose: To identify and describe adverse events (AEs) observed with real-world use of the following 3 vitrectomy platforms: Constellation (Alcon), Enhancing Visual Acuity (EVA, Dutch Ophthalmic Research Center), and Stellaris (Bausch + Lomb). Methods: All reports submitted to the US Food and Drug Administration's Manufacturer and User Facility Device Experience (MAUDE) database between January 2010 and November 2021 that were associated with the 3 vitrectomy platforms were analyzed. Each report was reviewed for AEs or consequences and the type of complication noted. Duplicates and reports with an inadequate narrative to categorize the event were excluded. A descriptive analysis was performed for the prevalence of device-specific issues within each platform. Results: The analysis included 2534 reports (1738 Constellation, 117 EVA, 679 Stellaris). Overall, the most commonly reported complications involved the vitrectomy probe (n = 957 [37.8%]) and the central processing unit (n = 368 [14.5%]). Differences in the distribution of AEs among the platforms were noted, with vitrectomy probe issues being the most reported events for the Constellation and Stellaris and infusion issues for the EVA. Infusion issues most frequently led to reports of patient harm with the Constellation (31/1738 [1.8%]) and EVA (7/116 [6.0%]), while issues with the vitrectomy probe were reported with the Stellaris (11/679 [1.6%]). Conclusions: An analysis of real-world data in the MAUDE database highlighted the spectrum of device-specific AEs of greatest relevance to surgical practice. Familiarity with potential device complications will help minimize harm to patients.
Keywords: Constellation; EVA; MAUDE database; Stellaris; adverse events; pars plana vitrectomy.
© The Author(s) 2024.
Conflict of interest statement
Dr. Kovas: Intergalactic Therapeutics (consultant). Dr. D’Amico: Alcon, Iveric Bio, and Aufbau Holdings (consultant); Iveric Bio and Aufbau Holdings (equity); Aufbau Holdings (intellectual property). Dr. Van Tassel: Alcon, Adverum, Abbvie, Equinox, New World Medical, Glaukos, Belkin, Bausch + Lomb, Zeiss, Character Biosciences, and Thea (consultant); Equinox (equity). Dr. Kiss: Adverum, Alcon, Novartis, Optos, and Genentech/Roche (consultant); Allergan, Novartis, Optos, Genentech/Roche, and Regeneron (research funding); Adverum, RegenxBio, and Fortress Bio (equity); gene therapy for age-related macular degeneration and T cells for CMV retinitis, assigned to Cornell University (intellectual property). Drs. D’Amico and Kiss reported that their consulting their consulting roles with Alcon had no bearing on the conception of this study, interpretation of the data retrieved from the MAUDE database, or composition of the manuscript. No discussion with third parties was held regarding the content of this manuscript, and the author(s) declared that their interpretation and analysis are solely their own. Authors with ongoing relationships with entities described in the manuscript were not involved in data acquisition, classification, or primary analysis. None of the other authors declared potential conflicts of interest with respect to the research, authorship, and/or publication of the article.
Figures
Similar articles
-
Analysis of Reported Adverse Events Associated with Sphincterotomes: An FDA Manufacturer and User Facility Device Experience (MAUDE) Database Study.Dig Dis Sci. 2025 Jul 24. doi: 10.1007/s10620-025-09224-3. Online ahead of print. Dig Dis Sci. 2025. PMID: 40707756
-
Safety and Procedural Success of Left Atrial Appendage Exclusion With the Lariat Device: A Systematic Review of Published Reports and Analytic Review of the FDA MAUDE Database.JAMA Intern Med. 2015 Jul;175(7):1104-9. doi: 10.1001/jamainternmed.2015.1513. JAMA Intern Med. 2015. PMID: 25938303
-
Adverse Events Associated with Devices for Incisional Glaucoma Surgery Performed with Implants as Reported to the FDA MAUDE Database.Graefes Arch Clin Exp Ophthalmol. 2025 Jun;263(6):1675-1679. doi: 10.1007/s00417-025-06771-3. Epub 2025 Feb 21. Graefes Arch Clin Exp Ophthalmol. 2025. PMID: 39979631 Free PMC article.
-
Eliciting adverse effects data from participants in clinical trials.Cochrane Database Syst Rev. 2018 Jan 16;1(1):MR000039. doi: 10.1002/14651858.MR000039.pub2. Cochrane Database Syst Rev. 2018. PMID: 29372930 Free PMC article.
-
Insights From the FDA's MAUDE Database Regarding the Real-World Safety of Jetstream Atherectomy for Peripheral Arterial Disease.J Endovasc Ther. 2025 Aug;32(4):1067-1074. doi: 10.1177/15266028231202718. Epub 2023 Sep 26. J Endovasc Ther. 2025. PMID: 37750495 Free PMC article.
References
-
- Machemer R, Buettner H, Norton EW, Parel JM. Vitrectomy: a pars plana approach. Trans Am Acad Ophthalmol Otolaryngol. 1971;75(4):813-820. - PubMed
-
- Machemer R, Hickingbotham D. The three-port microcannular system for closed vitrectomy. Am J Ophthalmol. 1985;100(4): 590-592. - PubMed
-
- Fujii GY, De Juan E, Jr., Humayun MS, et al. A new 25-gauge instrument system for transconjunctival sutureless vitrectomy surgery. Ophthalmology. 2002;109(10):1807-1812. - PubMed
-
- Fujii GY, De Juan E, Jr., Humayun MS, et al. Initial experience using the transconjunctival sutureless vitrectomy system for vitreoretinal surgery. Ophthalmology. 2002;109(10):1814-1820. - PubMed
-
- Khan MA, Kuley A, Riemann CD, et al. Long-term visual outcomes and safety profile of 27-gauge pars plana vitrectomy for posterior segment disease. Ophthalmology. 2018;125(3):423-431. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous