Which diagnostic test to use for Testing and Treatment strategies in Plasmodium vivax low-transmission settings: a secondary analysis of a longitudinal interventional study
- PMID: 39319096
- PMCID: PMC11420435
- DOI: 10.1016/j.lana.2024.100883
Which diagnostic test to use for Testing and Treatment strategies in Plasmodium vivax low-transmission settings: a secondary analysis of a longitudinal interventional study
Abstract
Background: The lack of sensitive field tests to diagnose blood stages and hypnozoite carriers prevents Testing and Treatment (TAT) strategies to achieve Plasmodium vivax elimination in low-transmission settings, but recent advances in Polymerase Chain Reaction (PCR) and serology position them as promising tools. This study describes a PCR-based TAT strategy (PCRTAT) implemented in Saint Georges (SGO), French Guiana, and explores alternative strategies (seroTAT and seroPCRTAT) to diagnose and treat P. vivax carriers.
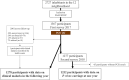
Methods: The PALUSTOP cohort study implemented in SGO (September 2017 to December 2018) screened participants for P. vivax using PCR tests and treated positive cases. Serology was also performed. Passive detection of P. vivax infection occurred during follow-up. Participants were categorised into overlapping treatment groups based on 2017 PCR and serological results. Strategies were described in terms of participants targeted or missed, primaquine contraindications (pregnancy, G6PD severe or intermediate deficiency), and sociodemographic characteristics.
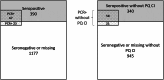
Findings: In 2017, 1567 inhabitants were included, aged 0-92 years. A total of 90 (6%) were P. vivax carriers and 390 seropositive (25%). PCRTAT missed 282 seropositive individuals while seroTAT would have missed 21 PCR-positive cases. Primaquine contraindications ranged from 12% to 17% across strategies.
Interpretation: Serology and PCR are promising tools for targeted treatment strategies in P. vivax low-transmission settings, when field compatible sensitive tests will be available. Both seem necessary to capture blood stages and potential hypnozoite carriers, while avoiding mass treatment. However, high primaquine contraindications rates need consideration for successful elimination.
Funding: Supported by European Funds for Regional Development, French Guiana Regional Health Agency, Pan American Health Organization, WHO, French Ministry for Research.
Keywords: Elimination; Low transmission; Plasmodium vivax; Relapse; Serology.
© 2024 The Author(s).
Conflict of interest statement
M.W. and I.M. are inventors on patent PCT/US17/67926 on a system, method, apparatus and diagnostic test for P. vivax. The other authors declare that they have no conflicts of interest.
Figures

Similar articles
-
[Role of primaquine in malaria control and elimination in French-speaking Africa].Bull Soc Pathol Exot. 2017 Aug;110(3):198-206. doi: 10.1007/s13149-017-0556-z. Epub 2017 Apr 17. Bull Soc Pathol Exot. 2017. PMID: 28417346 Review. French.
-
Developing sero-diagnostic tests to facilitate Plasmodium vivax Serological Test-and-Treat approaches: modeling the balance between public health impact and overtreatment.BMC Med. 2022 Mar 18;20(1):98. doi: 10.1186/s12916-022-02285-5. BMC Med. 2022. PMID: 35300700 Free PMC article.
-
Primaquine alternative dosing schedules for preventing malaria relapse in people with Plasmodium vivax.Cochrane Database Syst Rev. 2020 Aug 19;8:CD012656. doi: 10.1002/14651858.CD012656.pub3. Cochrane Database Syst Rev. 2020. PMID: 32816320
-
Asymptomatic Plasmodium falciparum and vivax infection in the neighborhood of Blondin, Saint-Georges-de-l'Oyapock District, French Guiana.Bull Soc Pathol Exot. 2017 Oct;110(4):265-269. doi: 10.1007/s13149-017-0572-z. Epub 2017 Sep 19. Bull Soc Pathol Exot. 2017. PMID: 28929395 English.
-
Accelerating towards P. vivax elimination with a novel serological test-and-treat strategy: a modelling case study in Brazil.Lancet Reg Health Am. 2023 May 19;22:100511. doi: 10.1016/j.lana.2023.100511. eCollection 2023 Jun. Lancet Reg Health Am. 2023. PMID: 37250687 Free PMC article.
Cited by
-
[Malaria control in French Guiana: What are the challenges in this last endemic French territory in 2024?].Med Trop Sante Int. 2025 Mar 20;5(1):mtsi.v5i1.2025.536. doi: 10.48327/mtsi.v5i1.2025.536. eCollection 2025 Mar 31. Med Trop Sante Int. 2025. PMID: 40248577 Free PMC article. French.
-
Parasitaemia and fever in uncomplicated Plasmodium vivax malaria: A systematic review and individual patient data meta-analysis.PLoS Negl Trop Dis. 2025 Mar 28;19(3):e0012951. doi: 10.1371/journal.pntd.0012951. eCollection 2025 Mar. PLoS Negl Trop Dis. 2025. PMID: 40153391 Free PMC article.
References
-
- WHO Guidelines for malaria. https://www.who.int/publications-detail-redirect/guidelines-for-malaria
LinkOut - more resources
Full Text Sources
Miscellaneous

