A comprehensive overview of recent progress in MXene-based polymer composites: Their fabrication processes, advanced applications, and prospects
- PMID: 39319124
- PMCID: PMC11419932
- DOI: 10.1016/j.heliyon.2024.e37030
A comprehensive overview of recent progress in MXene-based polymer composites: Their fabrication processes, advanced applications, and prospects
Abstract
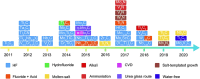
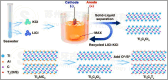
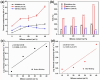
MXenes are a group of 2D transition metal carbonitrides, nitrides and carbides that have become widely recognized as useful materials since they were first discovered in 2011. MXenes, with their exceptional layered structures and splendid external chemistries, have excellent electrical, optical, and thermal properties, making them suitable for catalysis, biomedical uses, environmental remediation, energy storage, and EMI shielding. Over forty MXene compounds with surface terminations like hydroxyl, oxygen, or fluorine are hydrophilic and easily integrated into various applications. Advanced synthesis methods, including selective etching and etchant modifications, have broadened MXene surface chemistries for customized mechanical, thermal, and electrical applications. Integrating MXenes into polymer composites has demonstrated notable promise, enhancing the host polymers' electrical conductivity, thermal stability and mechanical strength. The MXene-polymer composites demonstrate remarkable prospective on behalf of advanced purposes, including flexible electronics, high-performance EMI shielding materials, and lightweight structural components. MXenes have the desirable characteristic of being able to create flexible and translucent films, as well as improve the properties of polymer matrices. This makes them very suitable for use in advanced technological applications. This review summarizes MXene research, methods, and insights, highlighting key discoveries and future directions. This also highlights the importance of ongoing research to fill in the gaps in current knowledge and improve the practical uses of MXenes.
Keywords: EMI shielding; MXenes; Nanofillers; Polymer composites; Protective coatings.
© 2024 The Author(s).
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures














Similar articles
-
A Comprehensive Review of Mxene-Based Emerging Materials for Energy Storage Applications and Future Perspectives.Chem Asian J. 2025 Feb 17;20(4):e202401181. doi: 10.1002/asia.202401181. Epub 2025 Jan 5. Chem Asian J. 2025. PMID: 39644135 Review.
-
MXene: fundamentals to applications in electrochemical energy storage.Discov Nano. 2023 Feb 3;18(1):3. doi: 10.1186/s11671-023-03786-9. Discov Nano. 2023. PMID: 36732431 Free PMC article. Review.
-
Recent advances in the development of MXenes/cellulose based composites: A review.Int J Biol Macromol. 2023 Jun 15;240:124477. doi: 10.1016/j.ijbiomac.2023.124477. Epub 2023 Apr 17. Int J Biol Macromol. 2023. PMID: 37076072 Review.
-
Structural evolution of MXenes and their composites for electromagnetic interference shielding applications.Nanoscale. 2022 Jul 7;14(26):9218-9247. doi: 10.1039/d2nr02224a. Nanoscale. 2022. PMID: 35726826 Review.
-
A Review on Multifunctional Polymer-MXene Hybrid Materials for Electronic Applications.Molecules. 2025 Apr 28;30(9):1955. doi: 10.3390/molecules30091955. Molecules. 2025. PMID: 40363762 Free PMC article. Review.
Cited by
-
Interface engineering of a MXenes/PVDF mixed-matrix membrane for superior water purification: efficient removal of oil, protein and tetracycline.RSC Adv. 2025 Aug 11;15(35):28413-28427. doi: 10.1039/d5ra03649f. eCollection 2025 Aug 11. RSC Adv. 2025. PMID: 40861974 Free PMC article.
-
Emerging Role of Nb2CTx MXene in Sensors: The Roadmap from Synthesis to Health and Environmental Monitoring.Sensors (Basel). 2025 Jun 12;25(12):3691. doi: 10.3390/s25123691. Sensors (Basel). 2025. PMID: 40573577 Free PMC article. Review.
References
-
- Lim K.R.G., Shekhirev M., Wyatt B.C., Anasori B., Gogotsi Y., Seh Z.W. Fundamentals of MXene synthesis. Nat. Synth. Aug. 2022;1(8):601–614. doi: 10.1038/s44160-022-00104-6. - DOI
Publication types
LinkOut - more resources
Full Text Sources

