Blood microRNA testing in participants with suspicious low-dose CT findings: follow-up of the BioMILD lung cancer screening trial
- PMID: 39319217
- PMCID: PMC11421266
- DOI: 10.1016/j.lanepe.2024.101070
Blood microRNA testing in participants with suspicious low-dose CT findings: follow-up of the BioMILD lung cancer screening trial
Abstract
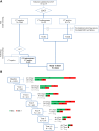
Background: The proper management of suspicious radiologic findings is crucial to optimize the effectiveness of low-dose computed tomography (LDCT) lung cancer screening trials. In the BioMILD study, we evaluated the utility of combining a plasma 24-microRNA signature classifier (MSC) and LDCT to define the individual risk and personalize screening strategies. Here we aim to assess the utility of repeated MSC testing during annual screening rounds in 1024 participants with suspicious LDCT findings.
Methods: The primary outcome was two-year lung cancer incidence in relation to MSC test results, reported as relative risk (RR) with 95% confidence interval (CI). Lung cancer incidence and mortality were estimated using extended Cox models for time-dependent covariates, yielding the respective hazard ratios (HR). Clinicaltrials.gov ID: NCT02247453.
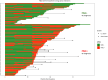
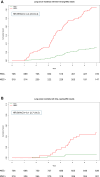
Findings: With a median follow-up of 8.5 years, the full study set included 1403 indeterminate LDCT (CTind) and 584 positive LDCT (CT+) results. A lung cancer RR increase in MSC+ compared to MSC- participants was observed in both the CTind (RR: 2.5; 95% CI: 1.4-4.32) and CT+ (RR: 2.6; 95% CI: 1.81-3.74) groups and was maintained when considering stage I or resectable tumors only. A 98% negative predictive value in CTind/MSC- and a 30% positive predictive value in CT+/MSC+ lesions were recorded. At seven years' follow-up, MSC+ participants had a cumulative HR of 4.4 (95% CI: 3.0-6.4) for lung cancer incidence and of 8.1 (95% CI: 2.7-24.5) for lung cancer mortality.
Interpretation: Our study shows that MSC can be reliably performed during LDCT screening rounds to increase the accuracy of lung cancer risk and mortality prediction and supports its clinical utility in the management of LDCT findings of uncertain malignancy.
Funding: Italian Association for Cancer Research; Italian Ministry of Health; Horizon2020; National Cancer Institute (NCI); Gensignia LifeScience.
Keywords: Biomarkers; Early detection; LDCT screening.
© 2024 The Author(s).
Conflict of interest statement
MBo, UP and GS are co-inventors of three patent applications regarding the miRNA signature classifier. These patents were licensed to a private company, Gensignia Life Science, under the regulations of Fondazione IRCCS Istituto Nazionale dei Tumori of Milan. All other authors declare no competing interests.
Figures
References
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

