Signal quality evaluation of an in-ear EEG device in comparison to a conventional cap system
- PMID: 39319310
- PMCID: PMC11420159
- DOI: 10.3389/fnins.2024.1441897
Signal quality evaluation of an in-ear EEG device in comparison to a conventional cap system
Abstract
Introduction: Wearable in-ear electroencephalographic (EEG) devices hold significant promise for integrating brain monitoring technologies into real-life applications. However, despite the introduction of various in-ear EEG systems, there remains a necessity for validating these technologies against gold-standard, clinical-grade devices. This study aims to evaluate the signal quality of a newly developed mobile in-ear EEG device compared to a standard scalp EEG system among healthy volunteers during wakefulness and sleep.
Methods: The study evaluated an in-ear EEG device equipped with dry electrodes in a laboratory setting, recording a single bipolar EEG channel using a cross-ear electrode configuration. Thirty healthy participants were recorded simultaneously using the in-ear EEG device and a conventional EEG cap system with 64 wet electrodes. Based on two recording protocols, one during a resting state condition involving alternating eye opening and closure with a low degree of artifact contamination and another consisting of a daytime nap, several quality measures were used for a quantitative comparison including root mean square (RMS) analysis, artifact quantification, similarities of relative spectral power (RSP), signal-to-noise ratio (SNR) based on alpha peak criteria, and cross-signal correlations of alpha activity during eyes-closed conditions and sleep activities. The statistical significance of our results was assessed through nonparametric permutation tests with False Discovery Rate (FDR) control.
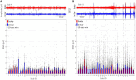
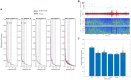
Results: During the resting state, in-ear and scalp EEG signals exhibited similar fluctuations, characterized by comparable RMS values. However, intermittent signal alterations were noticed in the in-ear recordings during nap sessions, attributed to movements of the head and facial muscles. Spectral analysis indicated similar patterns between in-ear and scalp EEG, showing prominent peaks in the alpha range (8-12 Hz) during rest and in the low-frequency range during naps (particularly in the theta range of 4-7 Hz). Analysis of alpha wave characteristics during eye closures revealed smaller alpha wave amplitudes and slightly lower signal-to-noise ratio (SNR) values in the in-ear EEG compared to scalp EEG. In around 80% of cases, cross-correlation analysis between in-ear and scalp signals, using a contralateral bipolar montage of 64 scalp electrodes, revealed significant correlations with scalp EEG (p < 0.01), particularly evident in the FT11-FT12 and T7-T8 electrode derivations.
Conclusion: Our findings support the feasibility of using in-ear EEG devices with dry-contact electrodes for brain activity monitoring, compared to a standard scalp EEG, notably for wakefulness and sleep uses. Although marginal signal degradation is associated with head and facial muscle contractions, the in-ear device offers promising applications for long-term EEG recordings, particularly in scenarios requiring enhanced comfort and user-friendliness.
Keywords: EEG; in-ear device; scalp EEG; signal quality; wearable technology.
Copyright © 2024 Moumane, Pazuelo, Nassar, Juez, Valderrama and Le Van Quyen.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures






Similar articles
-
Evaluating the Electroencephalographic Signal Quality of an In-Ear Wearable Device.Sensors (Basel). 2024 Jun 19;24(12):3973. doi: 10.3390/s24123973. Sensors (Basel). 2024. PMID: 38931756 Free PMC article.
-
Physiological artifacts in scalp EEG and ear-EEG.Biomed Eng Online. 2017 Aug 11;16(1):103. doi: 10.1186/s12938-017-0391-2. Biomed Eng Online. 2017. PMID: 28800744 Free PMC article.
-
Dry-Contact Electrode Ear-EEG.IEEE Trans Biomed Eng. 2019 Jan;66(1):150-158. doi: 10.1109/TBME.2018.2835778. Epub 2018 May 11. IEEE Trans Biomed Eng. 2019. PMID: 29993415
-
Advances in Electrode Materials for Scalp, Forehead, and Ear EEG: A Mini-Review.ACS Appl Bio Mater. 2023 Aug 21;6(8):3019-3032. doi: 10.1021/acsabm.3c00322. Epub 2023 Jul 26. ACS Appl Bio Mater. 2023. PMID: 37493408 Review.
-
The visual scoring of sleep and arousal in infants and children.J Clin Sleep Med. 2007 Mar 15;3(2):201-40. J Clin Sleep Med. 2007. PMID: 17557427 Review.
Cited by
-
Portable electroencephalography in early detection of depression: Progress and future directions.World J Psychiatry. 2025 Aug 19;15(8):107725. doi: 10.5498/wjp.v15.i8.107725. eCollection 2025 Aug 19. World J Psychiatry. 2025. PMID: 40837785 Free PMC article. Review.
References
-
- Benjamini Y., Hochberg Y. (1995). Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. B (Methodological) 57, 289–300. doi: 10.1111/j.2517-6161.1995.tb02031.x - DOI
-
- Berry R., Quan S., Abreu A. (2020). The AASM manual for the scoring of sleep and associated events: Rules, terminology and technical specifications, version 2.6. Darien: American Academy of Sleep Medicine.
-
- Butar B. F., Park J. W. (2008). Permutation tests for comparing two populations. J. Math. Sci. Math. Educ. 3, 19–30.
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

