Death-associated protein kinase 3 modulates migration and invasion of triple-negative breast cancer cells
- PMID: 39319326
- PMCID: PMC11421662
- DOI: 10.1093/pnasnexus/pgae401
Death-associated protein kinase 3 modulates migration and invasion of triple-negative breast cancer cells
Abstract
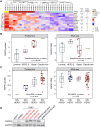
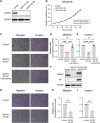
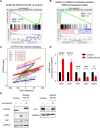
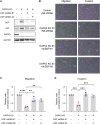
Sixteen patient-derived xenografts (PDXs) were analyzed using a mass spectrometry (MS)-based kinase inhibitor pull-down assay (KIPA), leading to the observation that death-associated protein kinase 3 (DAPK3) is significantly and specifically overexpressed in the triple-negative breast cancer (TNBC) models. Validation studies confirmed enrichment of DAPK3 protein, in both TNBC cell lines and tumors, independent of mRNA levels. Genomic knockout of DAPK3 in TNBC cell lines inhibited in vitro migration and invasion, along with down-regulation of an epithelial-mesenchymal transition (EMT) signature, which was confirmed in vivo. The kinase and leucine-zipper domains within DAPK3 were shown by a mutational analysis to be essential for functionality. Notably, DAPK3 was found to inhibit the levels of desmoplakin (DSP), a crucial component of the desmosome complex, thereby explaining the observed migration and invasion effects. Further exploration with immunoprecipitation-mass spectrometry (IP-MS) identified that leucine-zipper protein 1 (LUZP1) is a preferential binding partner of DAPK3. LUZP1 engages in a leucine-zipper domain-mediated interaction that protects DAPK3 from proteasomal degradation. Thus, the DAPK3/LUZP1 heterodimer emerges as a newly discovered regulator of EMT/desmosome components that promote TNBC cell migration.
Keywords: DAPK3; epithelial-to-mesenchymal transition; invasion; migration; triple-negative breast cancer.
© The Author(s) 2024. Published by Oxford University Press on behalf of National Academy of Sciences.
Figures


References
-
- Dent R, et al. 2007. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res. 13:4429–4434. - PubMed
-
- Manning G, Whyte DB, Martinez R, Hunter T, Sudarsanam S. 2002. The protein kinase complement of the human genome. Science. 298:1912–1934. - PubMed
-
- Engh RA, Bossemeyer D. 2002. Structural aspects of protein kinase control-role of conformational flexibility. Pharmacol Ther. 93:99–111. - PubMed
-
- Vogel CL, et al. 2002. Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer. J Clin Oncol. 20:719–726. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

