A breath-based in vitro diagnostic assay for the detection of lower respiratory tract infections
- PMID: 39319329
- PMCID: PMC11421151
- DOI: 10.1093/pnasnexus/pgae350
A breath-based in vitro diagnostic assay for the detection of lower respiratory tract infections
Abstract
An accurate diagnosis is critical to reducing mortality in people with lower respiratory tract infections (LRTIs). Current microbiological culture is time-consuming, and nucleic acid amplification-based molecular technologies cannot distinguish between colonization and infection. Previously, we described developing a sampling system for effectively capturing biomolecules from human breath. We identified a new class of proteoform markers of protease activation, termed proteolytic products of infection, for detecting LRTIs in people with mechanical ventilation. Here, we further developed an in vitro assay by designing a specific substrate sensor for human neutrophil elastase (HNE) to detect LRTIs in breath samples. In the proof-of-concept study, we then applied this in vitro assay to breath samples collected from intubated patients and healthy volunteers. The findings revealed that the LRTI group demonstrated a significant mean differential, showing a 9.8-fold elevation in measured HNE activity compared with the non-LRTI group and a 9.2-fold compared with healthy volunteers. The in vitro assay's diagnostic potential was assessed by constructing a receiver operating characteristic curve, resulting in an area under the curve of 0.987. Using an optimal threshold for HNE at 0.2 pM, the sensitivity was determined to be 1.0 and the specificity to be 0.867. Further correlation analysis revealed a strong positive relationship between the measured HNE activity and the protein concentration in the breath samples. Our results demonstrate that this breath-based in vitro assay provides high diagnostic performance for LRTIs, suggesting that the technology may be useful in the near term for the accurate diagnosis of LRTIs.
Keywords: human breath; lower respiratory tract infection; mass spectrometry; noninvasive diagnostics.
© The Author(s) 2024. Published by Oxford University Press on behalf of National Academy of Sciences.
Figures

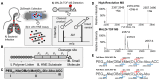
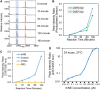
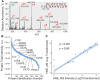
Update of
-
A Breath-Based In Vitro Diagnostics for Lower Respiratory Tract Infection.medRxiv [Preprint]. 2023 Sep 18:2023.09.18.23295728. doi: 10.1101/2023.09.18.23295728. medRxiv. 2023. Update in: PNAS Nexus. 2024 Sep 24;3(9):pgae350. doi: 10.1093/pnasnexus/pgae350. PMID: 37790344 Free PMC article. Updated. Preprint.
Similar articles
-
A Breath-Based In Vitro Diagnostics for Lower Respiratory Tract Infection.medRxiv [Preprint]. 2023 Sep 18:2023.09.18.23295728. doi: 10.1101/2023.09.18.23295728. medRxiv. 2023. Update in: PNAS Nexus. 2024 Sep 24;3(9):pgae350. doi: 10.1093/pnasnexus/pgae350. PMID: 37790344 Free PMC article. Updated. Preprint.
-
Application of mNGS in the Etiological Analysis of Lower Respiratory Tract Infections and the Prediction of Drug Resistance.Microbiol Spectr. 2022 Feb 23;10(1):e0250221. doi: 10.1128/spectrum.02502-21. Epub 2022 Feb 16. Microbiol Spectr. 2022. PMID: 35171007 Free PMC article.
-
Untargeted Molecular Analysis of Exhaled Breath as a Diagnostic Test for Ventilator-Associated Lower Respiratory Tract Infections (BreathDx).Thorax. 2022 Jan;77(1):79-81. doi: 10.1136/thoraxjnl-2021-217362. Epub 2021 Jun 4. Thorax. 2022. PMID: 34088787 Free PMC article.
-
Diagnostic value of the soluble triggering receptor expressed on myeloid cells-1 in lower respiratory tract infections: a meta-analysis.Respirology. 2014 May;19(4):501-7. doi: 10.1111/resp.12270. Epub 2014 Mar 24. Respirology. 2014. PMID: 24661408 Review.
-
Diagnostic accuracy of point-of-care tests for acute respiratory infection: a systematic review of reviews.Health Technol Assess. 2024 Oct 2:1-75. doi: 10.3310/JLCP4570. Online ahead of print. Health Technol Assess. 2024. PMID: 39359102
References
-
- Reddy KS. 2016. Global Burden of Disease Study 2015 provides GPS for global health 2030. Lancet. 388(10053):1448–1449. - PubMed
-
- Papan C, et al. 2018. Assessment of the multiplex PCR-based assay Unyvero pneumonia application for detection of bacterial pathogens and antibiotic resistance genes in children and neonates. Infection. 46:189–196. - PubMed
-
- Bonten MJ, Gaillard CA, de Leeuw PW, Stobberingh EE. 1997. Role of colonization of the upper intestinal tract in the pathogenesis of ventilator-associated pneumonia. Clin Infect Dis. 24(3):309–319. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

