Baseline MRI predictors of successful organ preservation in the Organ Preservation in Rectal Adenocarcinoma (OPRA) trial
- PMID: 39319400
- PMCID: PMC11422670
- DOI: 10.1093/bjs/znae246
Baseline MRI predictors of successful organ preservation in the Organ Preservation in Rectal Adenocarcinoma (OPRA) trial
Erratum in
-
Correction to: Baseline MRI predictors of successful organ preservation in the Organ Preservation in Rectal Adenocarcinoma (OPRA) trial.Br J Surg. 2024 Oct 30;111(11):znae302. doi: 10.1093/bjs/znae302. Br J Surg. 2024. PMID: 39566088 Free PMC article. No abstract available.
Abstract
Background: Prospective randomized trials have not yet identified baseline features predictive of organ preservation in locally advanced rectal cancers treated with total neoadjuvant therapy and a selective watch-and-wait strategy.
Methods: This was a secondary analysis of the OPRA trial, which randomized patients with stage II-III rectal adenocarcinoma to receive either induction or consolidation total neoadjuvant therapy. Patients were recommended for total mesorectal excision, or watch and wait based on clinical response at 8 ± 4 weeks after completing treatment. Standardized baseline clinical and radiological variables were collected prospectively. Survival outcomes, including total mesorectal excision-free survival, disease-free survival, and overall survival, were assessed by intention-to-treat analysis. Cox proportional hazards models were used to evaluate associations between baseline variables and survival outcomes.
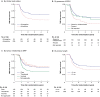
Results: Of the 324 patients randomized for the OPRA trial, 38 (11.7%) had cT4 tumours, 230 (71.0%) cN-positive disease, 101 (32.5%) mesorectal fascia involvement, and 64 (19.8%) extramural venous invasion. Several baseline features were independently associated with recommendation for total mesorectal excision on multivariable analysis: nodal disease (HR 1.66, 95% c.i. 1.12 to 2.48), extramural venous invasion (HR 1.57, 1.07 to 2.29), mesorectal fascia involvement (HR 1.45, 1.01 to 2.09), and tumour length (HR 1.11, 1.00 to 1.22). Of these, nodal disease (HR 2.02, 1.15 to 3.53) and mesorectal fascia involvement (HR 2.02, 1.26 to 3.26) also predicted worse disease-free survival. Age (HR 1.03, 1.00 to 1.06) was associated with overall survival.
Conclusion: Baseline MRI features, including nodal disease, extramural venous invasion, mesorectal fascia involvement, and tumour length, independently predict the likelihood of organ preservation after completion of total neoadjuvant therapy. Mesorectal fascia involvement and nodal disease are associated with disease-free survival.
© The Author(s) 2024. Published by Oxford University Press on behalf of BJS Foundation Ltd. All rights reserved. For commercial re-use, please contact reprints@oup.com for reprints and translation rights for reprints. All other permissions can be obtained through our RightsLink service via the Permissions link on the article page on our site—for further information please contact journals.permissions@oup.com.
Figures
References
-
- Maas M, Nelemans PJ, Valentini V, Das P, Rödel C, Kuo Let al. Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: a pooled analysis of individual patient data. Lancet Oncol 2010;11:835–879 - PubMed
-
- Martin ST, Heneghan HM, Winter DC. Systematic review and meta-analysis of outcomes following pathological complete response to neoadjuvant chemoradiotherapy for rectal cancer. Br J Surg 2012;99:918–928 - PubMed
-
- Zorcolo L, Rosman AS, Restivo A, Pisano M, Nigri GR, Fancellu Aet al. Complete pathologic response after combined modality treatment for rectal cancer and long-term survival: a meta-analysis. Ann Surg Oncol 2012;19:2822–2832 - PubMed
-
- Zwart WH, Temmink SJD, Hospers GAP, Marijnen CAM, Putter H, Nagtegaal IDet al. Oncological outcomes after a pathological complete response following total neoadjuvant therapy or chemoradiotherapy for high-risk locally advanced rectal cancer in the RAPIDO trial. Eur J Cancer 2024;204:114044. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

